Introduction
When considering the statistics of neoplastic diseases worldwide, breast cancer is found to have the highest incidence, followed by lung and colorectal cancer [1]. Breast cancer is present both in developed and in developing countries. Screening, early diagnosis, prompt treatment, and follow-up of the disease are all crucial. According to the Global Cancer Observatory (GLOBOCAN), 2,088,849 new cases of breast cancer occurred worldwide in 2018 [2]. For the same year, in Latin America and the Caribbean, the incidence was 27%, that is, more than 462,000 cases, according to the Pan American Health Organization [3]. Meanwhile, for 2019, the American Society of Clinical Oncology (ASCO) estimated 325,010 breast cancer cases in the United States, including in situ and invasive cancers [4]. The Spanish Association Against Cancer reported 33,307 new cases in 2019 [5]. In Colombia, an increase from 7,000 cases in 2012 to 13,380 in 2018 has been observed [2,6].
The highest mortality rates from cancer are attributed to lung, colorectal and stomach cancer, while breast cancer in women is in fifth place [1,2]. In 2018, there were 626,679 deaths from breast cancer worldwide, most frequently in Western Europe, East Asia and North Africa, with 169,640, 119,678 and 53,917, respectively [2,5]. In Latin America and the Caribbean, 14,097 deaths were identified, of which 3,702 occurred in Colombia [2].
Health care measures have reduced mortality and increased the rate of breast cancer survival [3,5], with survivors recovering productivity and returning to social and family interactions. More than 80% of women with early diagnosed breast cancer become over-10-year survivors [7]. Several variables influence survival: age, comorbidities, tumor extension, clinical stage at the time of diagnosis, time to the beginning of therapy, and presence of access barriers to health care, among others [4,8]. 12% lower five-year survival has been reported in women who had a delay of three months between diagnosis and the beginning of treatment, while 7% lower survival was recorded in those with delays of between three and six months [9]. Over the same number of years, 99% survival can be expected if the tumor is localized, 85% if it is regional, and 27% when there is distant invasion [4].
In addition, an increasing number of young women diagnosed with breast cancer have not yet completed their family [10]. Kopeika et al. [7] report that approximately 20% of localized or invasive breast cancers are diagnosed before menopause; the same was indicated by Del Mastro et al. [11]. There is uncertainty about the impact of the disease and its treatment on ovarian reserve or future reproduction, and about the impact of pregnancy on the survivor’s health [12,13]. The aim of this report is to present the case of a woman who, after 48 months of breast cancer survival, wished to carry a pregnancy, and also to indicate whether it is prudent to consent to or recommend pregnancy after a breast cancer diagnosis.
Case report
This report concerns a 46-year-old patient, Hispanic, physical therapist, living in Cartagena, Colombia. She had menarche at 12 years of age. Obstetric history: two pregnancies that ended by cesarean section and none miscarriages, both children born at term and exclusively breastfed until six months.
At 36 years of age, she perceived a mass in her left breast, associated with a burning sensation. On physical examination, the following were found: symmetrical breasts and a nodule of 1.6 centimeters in diameter in the lower left quadrant of the left breast, close to the submammary sulcus. Additionally, an elongated hardened area was palpated in the ipsilateral external superior quadrant. No lymphadenopathy, secretions or skin changes were identified. Breast ultrasound showed a homogeneous mass with a solid appearance. Mammography showed a heterogeneous fibroglandular mammary parenchyma, dense appearance of fatty tissue, and a spiculated nodular image. It was classified as BIRADS-V. Breast biopsy was performed and poorly differentiated infiltrating ductal carcinoma, nuclear grade-3 and equivocal test (2+) for the HER2/neu oncogene was identified.
Left breast quadrantectomy was performed, removing a firm, irregular, whitish mass measuring 2.5x2.0x3.0 cm. Sentinel node biopsy of the axillary region was performed. The histopathological report showed: “cells with abundant eosinophilic cytoplasm, nuclei with marked pleomorphism, vesicular, prominent nucleolus, and abundant atypical mitoses, arranged in a syncytial pattern, in solid nests. Stroma with moderate lymphocytic infiltrate, formation of cords and trabeculae with areas of necrosis and lymphovascular invasion”. The sentinel node study described: "follicular and sinusoidal hyperplasia, negative for malignancy". The pathological diagnosis was: "infiltrating, atypical medullary carcinoma with lymphovascular invasion". The pathological staging was T2N0M0, and the clinical staging IIA. Fluorescent in situ hybridization test (FISH) was positive for amplification of the HER2/neu oncogene. Immunohistochemical reaction showed: estrogen receptors in less than 2%, negative progesterone receptors and KI67 in 90% of all studied cells.
Thirty radiotherapy sessions, six anthracycline cycles, four paclitaxel cycles and one year of trastuzumab were performed. In addition, the left breast was esthetically reconstructed with transposition of the right abdominal rectum. During that period, family planning was based on fertility awareness-based and barrier methods. There were no changes in the frequency, duration or amount of menstrual bleeding during the course of the treatment or in the following three years.
At 41 years of age, with 48 months of breast cancer survival, the patient expressed the wish to have a new pregnancy. Treating gynecologists considered a new pregnancy neither opportune nor prudent, while her oncologists did not consider a new pregnancy to be risky. The patient, by her own decision, suspended family planning and, three months later, noticed a menstrual delay. On physical examination, a large, irregular, nodular uterus was found. hCG was positive, and on ultrasound an eight-week gestational sac with a live embryo plus multiple uterine fibroids were observed. Pregnancy was uneventful and ended at term, with a healthy newborn, who, at five years old, has shown normal growth and development.
At 120 months of survival, the patient has normal imaging follow-up and the tumor markers have been negative for recurrences. She has been asymptomatic, although in the last year she presented a right cervical nodule close to the jugular vein. A biopsy was performed and was negative for tumor cells.
Discussion
Worldwide there is an increasing trend to delay motherhood until the end of the third or beginning of the fourth decade of life [10,12]. This is consistent with an increase in the incidence of breast cancer in women who have not yet completed their family. In a series of Mexican survivors of breast cancer, 64.2% were nulliparous at the time of diagnosis [12]. Therefore, it is common for the desire for fertility to persist after the end of the treatment [10,13,14]. More than 45% of breast cancer survivors want to become pregnant [7].
There are several possible scenarios, since the reproductive impact of the treatment is not always predictable. In one of them, adjuvant therapy, which includes radiotherapy, chemotherapy or anti-estrogens, can cause gonadal cytotoxicity with apoptosis of ovarian germ cells [11,15]. Irregularity or absence of menstrual cycles, alteration in endocrine markers of integrity of the hypothalamic-pituitary-ovarian axis, early ovarian failure due to iatrogenic follicular depletion, early menopause, and subfertility are reported [10,16]. In these situations, assisted reproduction techniques with ovodonation will be required [11,15]. Goldrat et al. [17] observed no difference in rates of pregnancy, spontaneous or by assisted reproductive techniques, in breast cancer survivors.
In a second scenario, treatment-induced amenorrhea is temporary, depending on the drugs and doses used, and the patient’s age and organic conditions. Those under 35 years tend to achieve future fertility, facilitated by their increased ovarian reserve [7,12]. After breast cancer, the chance of achieving pregnancy with a live newborn is approximately 8% in women under 35 years of age and 3% in those aged 35-45 years [11]. Different ovulation induction schemes are available depending on the integrity of the ovarian cycles, but in some cases, spontaneous pregnancy is possible.
In a third scenario, menstrual cycles are not compromised and spontaneous pregnancies may occur [10,12,14,15,18], as happened in the patient described in this case report, who showed no changes in endocrinological reproductive function with the different oncological interventions performed.
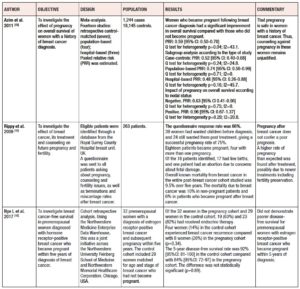
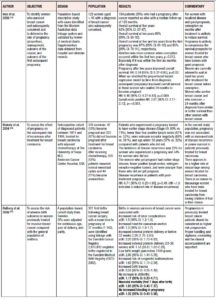
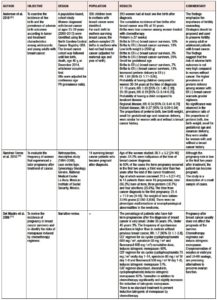
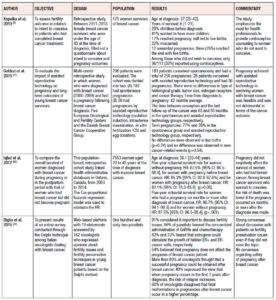
When health professionals, without distinction of specialty, are asked for an opinion or counseling by breast cancer survivors who want to achieve pregnancy, they will usually show some doubt or lack clear arguments [13,19]. Some of them, as well as the community in general, tend to mistakenly believe that pregnancy can be associated with breast cancer recurrence [7], however available clinical data do not show that women who become pregnant after a breast cancer diagnosis have a worse prognosis than those who do not [11,20]. Frequently, obstetricians and gynecologist have to respond if they consent to spontaneous pregnancy or if hormonal therapeutic tools or assisted reproduction techniques are needed to achieve it. Table 1 presents data that can be used to weigh up whether it is prudent to consent to or recommend pregnancy in breast cancer survivors.
The initial concern of survivors and their families is the risk/benefit relationship of a new pregnancy for the woman's health. It is a general cause of distress when the tumor expresses estrogenic receptors (+), since it is thought that high hormonal levels derived from pregnancy could induce cancer cell proliferation, tumor growth, recurrence and spread. However, several authors [13,18] indicate that lower expression of alpha estrogenic receptors, progesterone and HER2 can be expected in women who became pregnant after breast cancer compared with women who did not, so pregnancy could provide a protective effect in breast cancer survivors with estrogen receptors (+). In this subgroup of women, pregnancy can be consented after they have received adjuvant endocrine therapy, which is usually tamoxifen for between 5 and 10 years [18,21]. However, breast cancer may recur several years after primary therapy. Little is known about cancer cell biology in the survival stage, also called tumor latency. The estrogen receptor, the progesterone receptor, the receptor for human epidermal growth factor 2 (HER2), and the proliferation marker Ki-67 have been used for many years. They are used with the intent of predicting tumor prognosis and guiding therapy. Joensuu et al. point out that large tumor size and HER2 positivity are risk factors for rapid tumor recurrence, furthermore, positivity of the Ki-67 marker with a cut-off point of 14% was associated with early recurrence [22]. The applicability of tumor markers has been reinforced by the availability of genomic classification; DNA microarray profiles allow the identification of some breast cancer subtypes [23]. More studies are needed to help evaluate the interrelationship between tumor markers, genetic considerations, and survivor’s safety in a subsequent pregnancy.
It has been observed that long-term survival of patients who become pregnant after breast cancer is usually the same as or even longer than that of those who do not become pregnant. Also, survival is favored when conception occurs after 24 months from the diagnosis [14,19]. The patient described in the present report sought pregnancy 48 months after diagnosis. The frequency of spontaneous abortions is higher when pregnancy occurs in the first two years after diagnosis or during active treatment [24]. Another reason to avoid seeking pregnancy in the first two years is the greater possibility of recurrence [10]. In a meta-analysis of fourteen studies, Azim et al. [13] argue that this can be explained by breast remodeling during pregnancy, which increases angiogenesis, inflammation and extracellular matrix alterations, with stimulating effects on residual cancer cells, which previously were under therapy.
Rippy et al. [16], in a cohort of breast cancer survivors, found lower mortality at 60 months among those who became pregnant compared with those who did not, 6% and 10%, respectively. One of the hypotheses advanced to explain this better prognosis is the "healthy mother effect", which indicates that women who usually achieve pregnancy are those with early-stage tumors, early diagnosis, timely treatment, adequate psychosocial support, high self-esteem, high resilience, better prognosis, and no recurrences [11,14]. The present patient had all these elements in her personal, medical and family history. She was always aware of the inherent risks of pregnancy after the age of 40 years, but persisted in her desire for pregnancy. She also has a family and social support network.
Likewise, the fetal antigen theory, also known as alloimmunization, suggests the existence of protective immunization during pregnancy with antigens in fetal cells [13]. Fetal cells and breast cancer cells share common antigens, and there may be an immune response [13]. Additionally, it has been indicated that the decrease in estrogen levels at the end of pregnancy could induce apoptosis of abnormal mammary cells [18]. Igbal et al. [20] found that five years after the diagnosis of breast cancer, the survival rate was 96.7% among women who became pregnant, compared to 87.5% among those who did not. With the aforementioned indications, there seems to be no reason to forbid or discourage the desire for pregnancy [16,18,20]. However, there are not many pronouncements that recommend seeking pregnancy in order to improve or increase survival in women who have had breast cancer.
Biglia et al. carried out an expert consensus survey based on the Delphi methodology Italian oncologists to determine their opinion on aspects related to fertility in breast cancer survivors. 54% of oncologists agreed that pregnancy does not affect the prognosis of patients with breast cancer, while 49% reported that an increase in estrogen levels can stimulate growth of tumor cells. There was no consensus regarding opinions and recommendations on pregnancy. In clinical practice, too, there are discordant positions among specialists, as occurred in the case report presented. On the other hand, there was consensus among experts regarding the importance of breastfeeding and of discussing, with patients, the different ways of preserving fertility before cancer treatment [19].
Another concern frequently reported by survivors is the possibility of fetal or neonatal repercussions of oncological interventions [7,11]. In this regard, the most serious, and fortunately less frequent, are fetal congenital malformations. These can occur in 7% of cases, when the first trimester of pregnancy is concomitant with chemotherapy [15,25]. To reduce this risk, administration of folic acid prior to conception is suggested or, starting chemotherapy after the first trimester. The occurrence of fetal congenital malformations when pregnancy begins after completion of chemotherapy or radiotherapy treatment has not been documented [7,12,25].
Preterm delivery, low birth weight, small fetuses for gestational age, and cesarean delivery have been reported frequently in pregnant survivors of breast cancer [15,25]. However, Anderson et al. [21], in a cohort study based on data from North American populations, found that the proportions of these four outcomes were similar for women with and without a history of breast cancer.
Kasum et al. [10] indicated that abortion rates can be high, up to 29%, and that preterm deliveries with low birth weight can reach 40%. One consequence of radiotherapy is usually a decrease in the production of breast milk by the irradiated breast, due to atrophy of breast lobes [10,19]. No adverse outcomes or sequelae have been reported in newborns of breast cancer survivors [13].
It is important that health professionals, regardless of specialty, who monitor breast cancer survivors of reproductive age offer solid preconception counseling [7,16]. The important aspects to consider include: the patient’s age, the survival time, the treatment received, the time since completion, the tumor extension, tumor markers, and the presence of hormone receptors. Therefore, counseling should be individualized and aimed at achieving appropriate decision making. Several studies [14,16,17,18,20,21,24,25] and a meta-analysis [13] have emphasized that pregnancy is safe in breast cancer survivors.
If there is no reproductive desire, or if cancer therapy is ongoing, extensive contraceptive counseling should be provided. One study found that 66% of survivors did not use contraceptive methods, half of them did not want a pregnancy, and in turn 40% reported having regular menstrual cycles [7]. Recommendations should always be based on the medical eligibility criteria and suggesting methods with greater contraceptive efficacy. The opposite occurred with the patient on this case report, who used strategies with the greatest possibility of failure.
When diagnosing breast cancer in women of reproductive age, with or without children, it is important to establish whether they want to have children in the future [4,7,13,16,21]. Since a low pregnancy rate (3-8%) after breast cancer has been reported, and the probability of spontaneous abortions is high (over 20%) [11], patients should be sufficiently informed of the different options for fertility preservation that can be used before starting oncology therapy. Administration of luteinizing hormone-releasing hormone analogs, oocyte or embryo vitrification, and ovarian tissue cryopreservation are widely proposed alternatives [4,10,13,15,16,17,21]. Information in this regard was not provided to the patient whose case is described herein.
It is recommended, as routine practice, always to ask breast cancer survivors of reproductive age about fertility desire and family planning [7]. A multidisciplinary approach at the time of cancer diagnosis, involving gynecologists and oncologists, is important to offer optimal information on fertility preservation and future pregnancy possibilities [11,15]. The desire for pregnancy in breast cancer survivors should not be discouraged. However, further research on fertility and pregnancy are needed to adequately meet the requirements of female breast cancer survivors in the reproductive stage of life.
CONCLUSION
Important evidence indicates that it is safe to allow pregnancy, or to carry out interventions to achieve it, in breast cancer survivors. However, an individualized approach, taking into account the single patient’s personal conditions and oncological evolution, will always be necessary.
Interest conflict
None to declare
Financing
The Women's Health Research Group received financial and logistical resources and endorsement from Universidad de Cartagena to carry out this study, through the Strengthening and Sustainability Plan for Research Groups categorized by COLCIENCIAS. Act 064-2019 and Resolution 01430-2019. The authors received no financial resources for their participation in the research.