INTRODUCTION
It is crucial to promote the integration of the available information from the bench (biomedical science with its physiologic pathways) to bed side (practical clinical application of scientific developments).
The genital tract microbiome represents 9% of the total women’s microbiome.1 Recent advances in vaginal microbiome research have indicated that dysbiosis is a complex disorder involving not only cellular and bacterial metabolites, but also hormonal and environmental factors.
With newly acquired knowledge, bothersome and harmful gynecological conditions like Bacterial Vaginosis (BV) could be successfully treated to restore health and enhance quality of life across women’s lifespan.
Additionally, newest discoveries on Lactobacilli products and biofilms will allow us to address serious medical conditions. Predominantly related to pathogen biofilm producers and antibiotic resistant microorganisms like Pseudomonas aeruginosa which colonizes patients with severe infected burns and causes fatal sepsis.2
VAGINAL MICROBIOME
Having a better understanding of the vaginal microbiome is required to discern the pathogenesis of several ailments like Bacterial vaginosis.3
Accordingly, a multicenter epidemiological study started in China to analyze the vaginal microbiome by means of the Vaginal Microecology Evaluation System (VMES).4
Subsequently, innovative molecular techniques have permitted in-depth study of the vaginal microbiome. These includes DNA fingerprinting, microarrays, quantitative polymerase chain reaction (qPCR), genome sequencing (metagenomics), and bacteria specific sequencing 16S ribosome gene. Through these methods we have learned that the human vagina is a vastly nutrient-rich cavity for microorganisms (approximately 200 species has been reported) that bloom into a unique complex, dynamic, and diverse microbiome.5
The vaginal microbiome is under the effect of genetic, ethnic, hormonal, environmental, cultural and behavioral influences. This ecosystem in healthy women of childbearing age is habitually composed by 4 Lactobacilli that possess a specific vaginotropism: L. crispatus, L. jensenii, L. gasseri and L. iners.6 Vaginotropism is facilitated by pili that act as ligands for attachment to glycolipid receptors on vaginal epithelia cells.
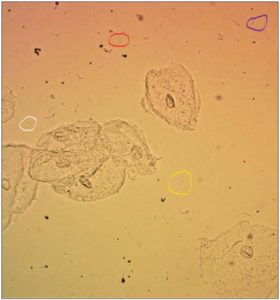
[Figure 1 recognizes the 4 most commonly Lactobacilli found in vagina in circles L. crispatus (blue), L. gasseri (white), L. iners (red) and L. jensenii (yellow). It is a wet mount microscopic image in normal saline solution at 200x magnification.]
Lactobacilli are obligate homo-fermenters of glucose from which they produce lactic acid that helps maintain a stable vaginal pH between 3.8 and 4.4. In addition, Lactobacilli produce hydrogen peroxide that allied with host myeloperoxidase and chloride ions, forms very powerful lethal oxidants that halt pathogenic bacteria from flourishing. Hydrogen peroxide also suppresses G. vaginalis and Mobiluncus. L. Crispatus and L. Jensenii are known to be hydrogen peroxide producers.7
Other products by Lactobacilli include organic acids, hydroxyl radicals, bio surfactants, arginine deaminases and biofilm.
Lactobacilli are likewise known to produce bacteriocins (ribosomal-produced antimicrobial peptides) which likewise defend the vaginal milieu from pathogen bacteria.8
Bacteriocins induce cell membrane permeabilization, altering ATR amino acids and ion efflux; therefore, transmembrane potential and provoke pH gradient depletion. Lactic acid potentiates bacteriocins and hydrogen peroxide.
Additionally, Bacteriocins express host cell immunity: NO hemolytic or cytotoxic activity. For instance, Lactocin 160A targets the cyto-membrane of G. vaginalis. 9
BACTERIAL VAGINOSIS
Bacterial Vaginosis (BV) is a vaginal disorder associated to a poly-microbial overgrowth of Gram-negative anaerobes and gram-positives bacteria, that displace most of Lactobacilli except sometimes L. iners.
Historical Synopsis
It was in 1955, when Gardner and Dukes identified a small gram negative bacillus in over 90% of women suffering from vaginitis which was termed Hemophilus vaginalis, since it was thought that bacterium to be the etiologic agent of this condition. 10 In their original research article, Gardner and Dukes also described in patients with this vaginitis, characteristic epithelial cells that presented distinctive stippled borders appearance by being covered with bacteria. 10, 11
Gardner and Dukes called them “Clue cells”, a name chosen for its conciseness in describing the essential of their identified vaginitis, today’s Bacterial Vaginosis. 10
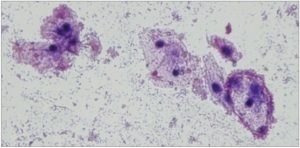
[Figure 2. Shows the typical “Clue Cells”: Bacteria obscuring the borders of vaginal epithelial cells, giving them a stippled appearance. Wet mount microscopic image in normal saline solution at 100x magnification with Gram Stain.]
A few years later it was found that Hemophilus vaginalis was gram-positive, therefore the microbiologists suggested that should be transferred to the genus Corynebacterium as Corynebacterium vaginalis (CV). Nevertheless, in 1977 Greenwood and Pickell informed that CV was both gram-intermediate and unrelated to the previously described Corynebacterium genera. Hereafter they elected to place it in a new genus, Gardnerella, as Gardnerella vaginalis.12
Bacterial Vaginosis Account
BV is a syndrome caused by communities of bacteria that include uncultivated species. BV could be clinically asymptomatic or be characterized by an increased thin gray or whitish vaginal discharge, with a foul, fishy like odor. Emotionally could be associated with embarrassment, uneasiness, and distress. There is a rise in vaginal pH from normal to 4.5 and over 5.5. Any or several of the more than the 180 species of bacteria present in the vagina may generate dysbiosis and BV. Especially when conditions alter, reduce, blocked, or deplete the dominant Lactobacilli. 13
The most commonly cultured bacteria in BV include: Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae, Mobiluncus spp, Bacteroides spp, Clostridiales spp, Peptostreptococcus spp, Prevotela spp, Fusobacterium, and Porphyromonas spp amongst others. 7, 14
The vaginal milieu comprises abundant microorganisms and the same environment provides them with nutrients for them to thrive. Disruptions in the vaginal balance within the microbiomes lead, to fluctuations in the vaginal environment, which increases the risk of acquiring sexually transmitted infections, and other bacterial and fungal infections. 15. 16
Bacterial vaginosis has been associated with premature rupture of membrane, preterm labor and birth, funisitis, chorio-amnionitis, post abortion infections, and with an increased risk of acquiring sexually transmitted diseases. 13
The variety of the vaginal microbiome relates to ethnicity and preterm birth tendency. 17 The incidence of preterm birth exceeds 10% worldwide. In the United States, BV and preterm birth have been more prevalent amongst non-Hispanic black women as compared to non-Hispanic white women. Women who births preterm showed significantly lesser vaginal concentrations of L. crispatus. 18, 19
Vaginal Immunity
It is puzzling that there is no clinical evidence of inflammation in BV patients despite the significant overgrowth of Gram-negative and Gram-positive anaerobes. Even with pro-inflammatory cytokines (IL-6, IL-8) present as reported by Spiegel et al in the vaginal secretions of these patients. 19
There are several theories to explain the absence of inflammation. The most accepted postulates that immunotolerance could occur as a result of coevolution between gut microbiome, innate, adaptive immunity, poor antibiotic penetration and/or alterations on bacterial physiology. 13
The immunotolerance is supported by short-chain fatty acids, with are known to modulate immune responses. These are produced in large amounts by anaerobe bacteria. 13
Vaginal immunity differs from systemic immunity. Reproductive tract immune cells safeguard against vaginal and cervical pathogens and establish immune tolerance for sperm and embryo/fetus in the uterus. The systemic immune system is under the influence of sexual hormones although the reproductive tract leukocytes do not have estrogen or progesterone receptors. 5, 15, 19
The innate immune system recognizes molecular patterns associated with pathogens (PAMP). When PAMP is recognized, it activates lymphocytes T and B causing pro-inflammatory cytokines release and the start of cellular and humoral immunity. The innate immune system acts rapidly but the acquired immunity system requires days.
Factors active in the vagina are mannose-binding lectin (MBL), complement and the membrane-associated components called Toll-like receptors (TLRs). MBL are antimicrobial protein synthesized in the liver. MBL binds to bacterial mannose-containing polysaccharide surfaces activate complement and cause bacteriolysis. The vagina also contains Defensins which are nonspecific antimicrobial activity molecules. Moreover, Secretory Leukocyte Protease Inhibitor (SLPI) destroys Gram-positive and
Gram-negative bacteria. These proteins are reduced in patients with BV. 5, 15, 19
Vaginal Epithelial Cells (VEC)provide an exclusive microenvironment that preserves vaginal health by nurturing endogenous Lactobacilli while holding acquired and innate immunity mediators. VEC hold in their surface complex Toll-like receptors (TLR). They recognize PAMP derived from various microbes. Eleven have been described, TLR1 and TLR2 distinguish in Gram-positive bacteria lipoproteins/peptidoglycans. [Vent] TLR4 recognizes liposaccharides in Gram-negative bacteria and TLR5 responds to flagellins in flagellated bacteria. And, TLR9 discriminates DNA sequences containing CpG dinucleotide exclusively in the non-methylated state (Humans DNA is highly methylated). 5, 15, 19
BIOFILMS
Biofilms are complex conglomerates in which bacterial populations live, are intertwined in, and thrive. Their formation is a sophisticated process that includes first, recognition of surface-related stimuli to enable adhesion and second, a production of a complex structure/function matrix. The matrix is formed of Extracellular Polymeric Substances (EPS). 20, 21
Recently, we have discovered about biofilms: there genetic determinants, the environmental conditions that influencing the biofilm formation and growth, and which bacteria are exhibiting biofilm-links traits. 22, 23
Additionally, how biofilms develop tolerance to antibiotic treatment and to host defenses. 4, 21
Biofilm formation by nonpathogenic bacteria is responsible for their maintenance and to help promoting the colonization and long-term permanence on the host’s vaginal mucosa.
For instance, G. vaginalis and A. vaginae develop biofilms while they colonize the vagina. This could explain the difficulty to eradicate them and the high recurrence of BV. 24
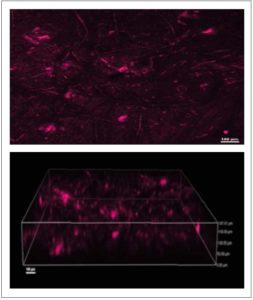
[Figure 3 Visualization of Gardnerella vaginalis biofilms. The biofilms were stained using Fluorescent in situ hybridization (FIH) staining and visualized using confocal laser scanning microscopy (CLSM). A) Two-dimensional image; B) Three-dimensional construction of the image in A.]
It has been estimated that the Biofilm formation potential by the ssp. which colonize the vagina are: 60 to 90% for G. Vaginalis, 1 to 40% for A. vaginae and only 1-5% by Lactobacilli. 25
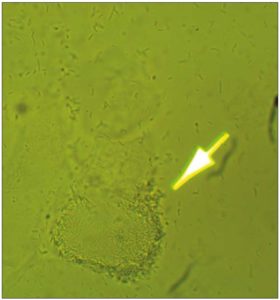
[Figure 4 is a microscopic photograph of biofilm produced by Lactobacilli. Wet mount in normal saline solution at 200x magnification.]
Terraf et al. isolated fifteen species of Lactobacillus from vaginal specimens and demonstrated that biofilm formation differed upon strain type, strain, culture medium, inoculum concentration, microbial growth and chemical nature of the support. 25
Lactobacilli exert a defensive role by interfering with pathogenic bacterial growth and/or adhesion. 6 Biofilm formation by Lactobacilli in vivo has been reported in saline wet mount preparations from uninfected vaginal samples. 26
Pathogen biofilm producers and antibiotic resistant microorganisms like Pseudomonas aeruginosa frequently infects patients with severe infected burn wounds leading to sepsis, multiple organ failure and death.
Lactobacilli spp. has shown in vitro to inhibit the growth and biofilm development by P. aeruginosa. 26, 27
Although more importantly, a recent report by our research team has showed in an animal model that 20× concentrated supernatant from L. gasseri inhibited the growth of P. aeruginosa, prevented biofilm development and partially eliminated already developed biofilms. 2 These results suggest a potential use of L. gasseri in preventing sepsis from P. aeruginosa infection in severely burned and immunocompromised patients. 2
Unquestionably, these newest reports and discoveries on Lactobacilli products and biofilms will allow us to appropriately address these serious medical conditions. 28
CONCLUSIONS
Vaginal microbiome research has revealed that dysbiosis is a complex disorder involving cellular and bacterial metabolites, hormonal, and environmental factors. This understanding has helped to address Bacterial Vaginosis research and progress toward effective prevention and treatment. Furthermore, newest discoveries on Lactobacilli products have shown that L. Gasseri inhibited the growth and biofilm production of P. aeruginosa in an animal model suggesting a potential use of it in preventing sepsis in severely burned and immunocompromised patients.
Conflict of Interest: No conflict of interest from any of the 2 authors.
All the figures are originals from the authors.