Introduction
Since the 18th century, researchers and scientists have sought to examine the diversity of bacteria that inhabit the vaginal cavity. A variety of methods have been used from light microscopy and gram staining to more recently, bacterial culturing and polymerase chain reaction (PCR). These and other modern techniques have allowed scientists to study the microbial composition and to identify the specific Lactobacillus species present (1). The occurrence of Lactobacilli as the majority species found in the vaginal flora has been associated with a healthy vaginal milieu. The most prevalent species in a healthy vagina of women of child-bearing age are Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus iners. Under the influence of estrogen, glycogen from vaginal epithelial cells is utilized by Lactobacilli to produce lactic acid, which in turn, lowers the pH of the vagina, thus, making the environment less favorable for the growth of pathogenic bacteria (2). Fluctuations in a woman’s life can cause the balance of Lactobacilli to shift from high colonization to low colonization. Normal female life events such as ging, menstruation, sexual activity, hygiene, medications, and estrogen levels can affect the vaginal milieu. Consequently, a shift toward increased numbers of non-Lactobacillus bacterial species results in dysbiosis that is associated with bacterial vaginosis (BV) (3). BV is the leading cause of abnormal vaginal discharge, leading to more than 13.5 million visits to healthcare providers each year (4). BV has been associated with adverse obstetric and gynecological outcomes in women. These complications include an increased risk of acquiring sexually transmitted infections, and developing pelvic inflammatory disease, increased risk of miscarriage, preterm labor and delivery, as well as an increased risk of post-delivery complications including wound infections and postpartum endometritis (5). The vaginal bacterial communities of post-menopausal women show significantly decreased L. spp. (3). Menopause is defined as the permanent cessation of menstruation due to loss of ovarian function, clinically diagnosed after 12 months of amenorrhea (1). The average age of menopause is largely determined by genetic factors (6). For some women, menopause can be accompanied by hot flashes, vaginal dryness and atrophy, loss of bone density, weight gain, sexual dysfunction, and mood alterations (1). Additionally, the vaginal microbiome of post-menopausal women experiences a major shift, caused by a decrease in circulating estrogen (1).
Vaginal, ectocervical and endocervical epithelial cells secrete regulatory factors (chemokines and cytokines), antibacterial products (lactoferrin, lysozyme, complement, and defensins) and immunoglobulins A and G, in order to protect themselves. Cervical secretions include cytokines IL-1β, IL-6, IL-10, IL-18, CC-chemokine ligand 2, and vascular endothelial growth factor (VEGF). Cytokines modulate assorted physiological, inflammatory, and non-inflammatory processes (7).
The onset of menopause is related to a low systemic inflammatory status, manifested by increased levels of several different cytokines. Menopause has been known to spark changes in the activity of pro-inflammatory cytokines. The activated cells, which secrete cytokines, are less present in post-menopausal women when compared to fertile women, and the activity of these cells did not correlate with sex hormone levels (8). As we can tell from previous studies, L. spp. present in pre-menopausal and post-menopausal women have not been associated with the presence of inflammatory cytokines in the vaginal milieu that could be utilized as biomarkers to distinguish vaginal health from vaginal dysbiosis.
Objective
The study objective is to evaluate the association between vaginal Lactobacilli and cytokine levels in pre-menopausal and post-menopausal women.
Materials and methods
The vaginal samples originated from asymptomatic (no vaginal discharge and no vaginal symptoms) of pre-menopausal and post-menopausal patients attending a specialized university based outpatient vulvovaginal clinic. These patients consulted the clinic exclusively for vulvar non-infectious conditions.
Vaginal swabs were collected as part of a database collection – prospective data bank creation, to study vaginal conditions which were IRB approved (IRP protocol# L13-054) from Texas Tech University Health Sciences Center, Odessa, TX, USA. The samples in the database were obtained from the middle of the vagina using standardized cotton swabs. No other vaginal areas were sampled (9). Vaginal specimens were placed into 1 ml of physiological solution (phosphate-buffered saline) and stored at -80 0C.
Real-Time PCR (qPCR)
The relative concentration of the vaginal flora was determined by real-time PCR (qPCR), as described previously (10, 11). The qPCR assay was performed to identify vaginal Lactobacillus spp., including L. crispatus, L. gasseri, L. iners, and L. jensenii. In addition, the presence of facultative anaerobic bacteria (Gardnerella vaginalis, Atopobium vaginae, Megasphaera spp., Eggerthella spp., Prevotella spp., Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium) was also determined. qPCR analysis of gene transcripts was performed using a BioRad iCycler RealTime PCR machine, 2X Taqman Master Mix. For RNA preparation, samples were processed using the TRIzola (Invitrogen, Carlsbad, CA) method. qPCR data were analyzed using the comparative ∆∆Ct method (10).
Cytokine estimation
Cytokine analysis was performed using the Bio-Plex MAGPIX multiplex reader instrument (Bio-Rad, USA) and the MesoQuick Plex SQ 120 instrument (Meso Scale Discovery, MD, USA).
1) The Bio-Plex Pro Human cytokine 27-Plex Immunoassay is a 96-well kit (Cat#M500KCAF0Y, Bio-Rad, USA) that includes magnetic beads, detection antibodies, wash buffer, sample diluent, detection antibody diluent, a 96-well flat bottom plate, and a plastic adhesive plate seal. This assay detects a total of 27 cytokines in the samples. The 96-well plate was pre-wetted with 100 µl of Bio-Plex assay buffer. 50 µl of working bead solution was added into each well. The plate was washed with 100 µl of Bio-Plex wash buffer (2X). Then, 50 µl of standards and 50 µl of samples were added to the appropriate wells of the plate. The plate was covered with a plastic adhesive plate seal (to block out light) and incubated for 30 min at room temperature with shaking. The plate was then washed with 100 µl of Bio-Plex wash buffer (3X). 25 µl of Bio-Plex detection antibody diluent was added to each well of the plate. The plate was again covered with a plastic adhesive plate seal and incubated for 30 min at room temperature with shaking. Next, the plate was washed with 100 µl of Bio-Plex wash buffer (3X). 50 µl of Streptavidin-PE (working dilution 100X) was added to each well of the plate. The plate was covered with a plastic adhesive plate seal and incubated for 10 min at room temperature with shaking. Again, the plate was washed with 100 µl of Bio-Plex wash buffer (3X).
The beads were re-suspended in each well with 125 µl of Bio-Plex assay buffer. The plate was covered with a plastic adhesive plate seal and placed on a shaker for 30 seconds at 1100 rpm. Finally, the plastic adhesive plate seal was removed and the plate was immediately read using the Bio-Plex MAGPIX multiplex reader instrument (USA).
2) MSD (Meso Scale Discovery) cytokine assays provide a rapid and convenient method for measuring the levels of cytokines within a single, small volume sample. An MSD 96-well plate was pre-coated with capture antibodies on independent and well-defined spots. All vaginal swab samples were analyzed using the MSD multiplex instrument MESO QuickPlex SQ 120 (MSD, MD, USA). The MSD electrochemiluminescence (ECL) detection system has been validated for cytokine measurement in vaginal swab samples (12).
A total of 5 custom plates were made for the multiplex assays:
1) Plate 1: IFN-γ (Interferon – γ), IL-1β (Interleukin – 1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α (Tumor necrosis factor – α)
2) Plate 2: GM-CSF (Granulocyte-macrophage colony-stimulating factor), IL-5, IL-7, IL-15, IL-17A
3) Plate 3: Eotaxin, MIP-1α (Macrophage inflammatory protein - 1α), MIP-1β, MCP-1 (Monocyte chemoattractant protein -1)
4) Plate 4: VEGF-A (Vascular endothelial growth factor - A), bFGF (Basic fibroblast growth factor)
5) Plate 5: IL-1RA, IL-9.
The whole kit, including calibrators, controls, samples diluent, wash buffer as well as read buffer, detection antibody solution and a 96-well plate (Cat # N05049A-1, MSD, USA) was provided by MSD to perform multiplex assays and to detect a total of 23 cytokines in the samples. The plate was washed 3 times with 150 µl/well of wash buffer. 50 µl of samples, calibrators, or controls were added into each well of the plate. The plate was sealed with an adhesive plate seal and incubated at room temperature for 2 h on a shaker at 700 rpm. The plate was then washed 3 times with 150 µl/well of wash buffer. 25 µl of detection antibody solution was added to each well, sealed and incubated at room temperature for 2 h on a shaker at 700 rpm. The plate was then washed 3 times with 150 µl/well of wash buffer. 150 µl of read buffer
was added to each well. Finally, the plate was analyzed using the MSD multiplex instrument.
Statistical Analysis
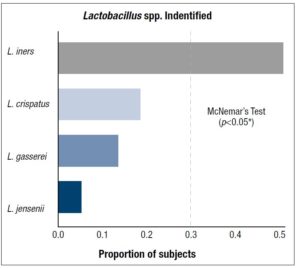
The significance level for inference was set at α = 0.05. Statistical analysis was completed using GraphPad Prism with R programming. Fisher’s test was used to compare proportions of pre-menopausal and post-menopausal women with individual Lactobacillus spp. Proportions for different Lactobacillus spp. identified in subjects irrespective of menopause was compared using McNemar’s test for dependent samples with the Holm-Bonferroni method for multiple comparisons used for adjusted p-values. Exploratory analysis using normal quantile plots show that cytokine data are drawn from non-normal populations with boxplots showing that cytokine data is right skewed with means greater than medians and the presence of large outliers. The median was selected as the measure of central tendency for inference. The data was grouped by presence of individual Lactobacillus spp. and differences in cytokine levels analyzed using the
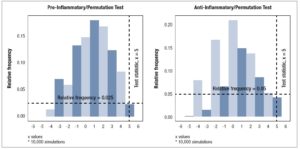
Mann-Whitney test. The Mann-Whitney test was also applied to individual cytokine data grouped into pre-menopause and post-menopause subjects. Associations within and between cytokine classifications were analyzed using Spearman’s rank-correlation test. Permutation tests based on the randomization of menopause classification with10,000 simulations were used to test the hypothesis that joint distributions of cytokines are the same in pre-menopausal and post-menopausal women with the test statistic being the sum of the signs of the differences of the group medians. The permutation test was applied to pro-inflammatory cytokines (IL-1β, IL-2, IL-6, IL-12p70, IL-17A, IFN-γ, TNF-α), anti-inflammatory cytokines (IL-4, IL-5, IL-6, IL-10, IL-13, IL-1RA), chemokines (IL-8, IP-10, MIP-1α, MIP-1β, MCP-1, RANTES), growth factors (PDGF-BB, VEGF-A, bFGF), cytokines of eosinophil recruitment and activation (IL-2, IL-4, IL- 5, GM-CSF, RANTES, Eotaxin), and cytokines of neutrophil recruitment and activation (IL-1β, IL-17A, TNF-α, G-CSF).
Results
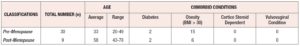
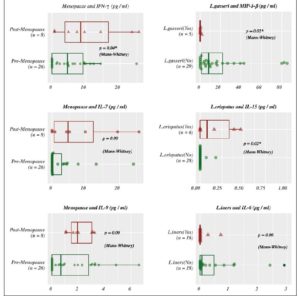
The average age of pre-menopausal patients was 33 years and the average age of post-menopausal patients was 58 years. Post-menopausal patients showed higher comorbid conditions including diabetes and obesity (BMI>30) compared to pre-menopausal patients (Supplementary table 1). Regarding the vaginal microbiome we found significant evidence that L. iners was the most abundant species followed by L. gasseri and L. jensenii (*p < 0.05) (Figure 1, Table 1). In addition, we found significant associations between L. species identified and cytokine levels, with the level of MIP-1-β higher in patients identified with L. gasseri compared to patients where L. gasseri was not detected, and the level of IL-15 was higher in patients identified with L. crispatus compared to patients that did not exhibit the presence of L. crispatus (*p < 0.05) (Table 2, Supplementary Table 2). We also found significant evidence that the level of IFN -γ was higher in post-menopausal patients compared to pre-menopausal patients (*p < 0.05) (Figure 2, Table 3).
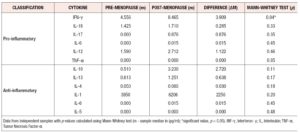
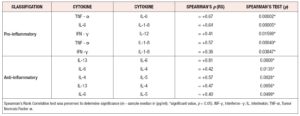
For both pro-inflammatory and anti-inflammatory cytokines, five of the six cytokines that were measured had a higher median concentration in post-menopausal patients, with one cytokine having the same median concentration, and no cytokines having a lower median concentration in pre-menopausal patients. Moreover, we found significant evidence that cytokine levels were correlated with positive associations between the anti-inflammatory cytokines IL-4, IL-6 and IL- 13 and positive associations between the anti-inflammatory cytokines TNF-α, IL-1-β, and IL-6 identified (*p < 0.05) (Figure 3, Table 4).
Out of the 26 cytokines analyzed, 19 cytokines showed higher levels of sample median concentrations in the post-menopausal group than the pre-menopausal group. Only four cytokines showed the same sample median concentrations, and three cytokines showed a decrease in sample median concentrations in post-menopausal patients.
For both pro-inflammatory and anti-inflammatory cytokines, five of the six cytokines that were measured had a higher median concentration in post-menopausal patients, with one cytokine having the same median concentration, and no cytokines having a lower median concentration in post-menopausal subjects. Furthermore, we found significant evidence via permutation test that pro-inflammatory and anti-inflammatory cytokine levels were higher in post-menopausal patients compared to pre-menopausal patients (*p < 0.05) (Figure 4, Table 5).
Discussion
Out study results demonstrated that there was a direct correlation between Lactobacillus species and cytokine expression, and the median concentrations of pro-inflammatory and anti-inflammatory cytokines were elevated in post-menopausal women compared to their pre-menopausal counterparts. Some research studies have confirmed that the vaginal microbiome and cytokine profile changes with age (13-15). Our study results demonstrated that IL-1RA showed a significant difference between pre-menopausal and post-menopausal women. The results of our study are directly correlated with previous findings that IL-1β, IL-6, IL-8 and TNF-α were expressed highly in post-menopausal women (6).
In agreement with the literature, pro-inflammatory cytokines IL-1α, IL-1β, IL-8 and anti-inflammatory cytokine IL-1RA were considered vaginal epithelial or mucosal damage biomarkers in post-menopausal women (16). Free glycogen in vaginal fluid is released from epithelial cells by enzymes like α-amylase which associated with Lactobacillus colonization in the vagina of pre-menopausal and post-menopausal women, suggesting that glycogen might be a mediating factor for this bacterial species presence (2, 7).
Our study displayed that a pro-inflammatory cytokine, IL-6, was expressed slightly higher in post-menopausal women compared to pre-menopausal women. IL-6 is a potent mediator of inflammatory processes; therefore any increase in circulating IL-6 levels might be of relevance to the etiology of a number of age-related clinical disorders (6). Increased levels of pro-inflammatory cytokines and changes in levels of anti-inflammatory cytokines in post-menopausal women may be associated with monocyte and macrophage function, which is impaired due to estrogen deficiency (17).
Our findings revealed that only L. iners and L. gasseri were identified in the post-menopausal group. L. spp. were identified in smaller amounts in post-menopausal women compared to pre-menopausal women. In agreement with the results that vaginal bacterial communities of post-menopausal women were usually not dominated by L. spp. (3). Recent research showed that the large presence of L. iners could be related to its evolution in the genus which resulted in having the smallest DNA of the L. spp., through gene transfer and acquisition of foreign DNA which made L. iners more competitive to the vaginal environment (18).
Our study results showed that TNF-α cytokine level was elevated in vaginal swabs of the post-menopausal group compared to the pre-menopausal group. In agreement with the literature, TNF-α contributes to the development and progression of atherosclerosis and osteoporosis. The increase in TNF-α levels in post-menopausal women may explain the pathogenesis of postmenopausal symptoms (19).
Our findings show that IFN-γ had a statistically significant increase in post-menopausal women (m=8.465) with a proportional increase almost double that of pre-menopausal women (m=4.556). IFN-γ levels do not necessarily change in relation to sex hormone levels. They do, however, vary based on the level of dehydroepiandrosterone sulfate (DHEA-S).
In other studies, IFN-γ levels are normally present in elevated levels in pre-menopausal women, but have been shown to be present in similar levels in pre-menopausal women and post-menopausal women who are on hormone-replacement therapy, suggesting sex hormones such as estrogen could cause a basal increase in IFN-γ, apart from DHEA-S facilitated production (8). In agreement with the literature, IFN-γ is an important cytokine for its effects on the immune system, enhancing antigen presentation and macrophage activation, secreted by both, T-helper cells 1 and 2 (Th1 &Th2), and functions to activate immunity to bacteria and viruses (20). Increased levels of IFN-γ has been associated with have a role in promoting osteoblast differentiation (20). The elevated levels of IFN-γ present in post-menopause could suggest an explanation for the increase in osteoporosis which is common among post-menopausal women.
The main limitation of our research study is that we used multiplex cytokine immunoassays instead of more high-sensitivity assays. Another limitation of the study is small sample sizes in both groups.
Abbreviations:
| bFGF | Basic fibroblast growth factor | |
| BV | Bacterial Vaginosis | |
| DHEA-S | Dehydroepiandrosterone sulfate | |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor | |
| IFN | Interferon | |
| IL | Interleukin | |
| MCP | Monocyte chemoattractant protein | |
| MIP | Macrophage inflammatory protein |
| MSD | Meso Scale Discovery | |
| PDGF | Platelet derived growth factor | |
| qPCR | Real time polymerase chain reaction | |
| RANTES | Regulated on activation, normal T cell expressed and secreted | |
| Th | T helper | |
| TNF | Tumor necrosis factor | |
| VEGF | Vascular endothelial growth factor | |
Acknowledgements:
We are grateful to the Texas Tech University Health Sciences
Center, Permian Basin, TX, and Cathy Lovett, Ailena Mulkey, Evangelina Santiago,
and Jammie Holland from Clinical Research Institute, TX for their excellent
support on this study. We also appreciate the great help of Elea Stout, an undergraduate
student from the University of Texas of the Permian Basin, in the manuscript
editing. We value the kind efforts of Mr. Erik Wilkinson (Regional Director of
the TTUHSC – Library of the Health Sciences at the Permian Basin, Odessa, TX)
in the literature search and review. In addition, we would like to acknowledge Dr.
Scott Gygax and his team for his assistance in the quantification of the vaginal
flora by qPCR analysis.
Conflict of interest:
The authors declare that they have no conflict of interest.