Introduction
In Italy infertility is estimated to affect about 20% of couples who, failing to achieve a pregnancy, resort to assisted fertilization technology. Depending on the cause of infertility, a couple can access homologous or heterologous fertilization techniques. The former involve the use of the couple's own gametes, while heterologous fertilization techniques involve the use of gametes (sperm or oocytes) from donors.
Trounson reported the first successful creation of a pregnancy from a donated oocyte, and this development changed the course of human reproduction (1). Whereas egg donation was initially reserved for women with premature ovarian failure or with no ovaries, nowadays it is mainly used to treat couples with multiple IVF failures and with advanced maternal age.
The need for donated oocytes has increased in the last decade as demonstrated, globally, by the high number of donor oocyte cycles carried out (133679 between 2008 and 2010) (2).
In 2016, according to the Italian National Register of Assisted Reproduction Technology, 2674 patients received oocyte donation with 2901 cycles; 880 pregnancies were reported, resulting in 647 live-born babies.
In Italy, “couples over 18, married or cohabiting, of potentially fertile age, both living” in whom a disease that causes irreversible sterility or infertility in one or both partners has been established and certified, may have access to heterologous fertilization techniques (Art. 5, Law 40/2004).
As established by the Conference of the Regions and Autonomous Provinces, the donation must be anonymous and donor gametes may not generate more than 10 births. The donation of male and female gametes must be voluntary and must not entail any economic remuneration. Male donors must be aged between 18 and 40, while female donors must be between 20 and 35 years of age.
The difficulty finding donors in our country makes it increasingly difficult to cope with the high number of requests; for this reason, we normally turn to gamete banks and, consequently, vitrified oocytes.
In Italy we have gained a good experience in this field due to the ban on freezing embryos (Law 40 of 2004). As an alternative, and in accordance with the legal prohibition of embryo cryopreservation, oocyte vitrification was introduced into routine practice in Italy (3-7). It was not until 2009 that the Italian Constitutional Court (judgement 151/2009) declared the constitutionality of embryo cryopreservation. Moreover, oocyte vitrification has also emerged as an important method for female fertility preservation, for both medical and non-medical indications (8, 9).
There are conflicting opinions concerning the quality of vitrified oocytes. Cobo et al. reported no differences in ongoing clinical pregnancy between fresh and vitrified oocytes (10).
Three separate studies revealed no evidence of a difference in live-birth rate (LBR) between cycles using exclusively vitrified versus fresh oocytes in live-birth rate (11-13), as a retrospective cohort study from the National ART Surveillance System does.
Conversely, a retrospective analysis of 2013 through 2015 aggregate US national data including 30160 IVF cycles with either fresh or vitrified donor oocytes indicated that “fresh donor oocytes produced significantly higher live birth rates per recipient cycle start than vitrified donor oocytes”, concluding that fresh oocyte donation must, therefore, still be considered the ‘gold standard’ in oocyte donation (14).
In Italy, in April 2014, the Constitutional Court (judgement n.162/2014) condemned as unconstitutional the ban of heterologous fertilization, thereby allowing many infertile Italian couples wider access to assisted reproductive treatments, without the need to seek them abroad.
Under Italian law, all couples in whom competent own gametes cannot be obtained from one of the two partners, or both, may have access to oocyte (or sperm) donation. Another indication is severe isoimmunization of the female partner, may occur when she is Rh-negative and her male partner is Rh-positive.
Due to the shortage of gamete donors in Italy, the use of vitrified oocytes is currently the most widely used option.
The use of vitrified oocytes raised the following questions: how many oocytes should be used per patient? How many oocytes give the best pregnancy rate?
Only limited literature has analyzed this aspect, with allocation policies mainly found to range from a minimum of 4 oocytes to a larger number, such as 8 or 10 oocytes. Obviously, this decision is very important in cost-effectiveness terms, therefore the objective of our study was to analyze how reproductive outcomes vary according to the number of oocytes allocated.
In our observational study we evaluated fertilization rate and pregnancy rate in women undergoing ART treatments with donated oocytes, dividing them into two groups according to the number of oocytes retrieved (3, 4, 5 oocytes vs 6, 7, 8 oocytes).
To date, the costs of obtaining gametes are borne by the couple and depend on the number of oocytes. For this reason, in our center, we give couples the possibility to choose between smaller or larger sets (mini-lots or complete lots) of oocytes.
It is important to find a way of ensuring that heterologous fertilization techniques are accessible in a uniform manner throughout the national territory in order to protect the right to parenthood of all couples who wish to form a family.
Materials and Methods
This is a retrospective observational study of anonymized donor oocyte IVF cycles with elective vitrified-thawed oocytes shipping and from January 2016 to July 2019 between donor clinics in Spain and A.G.I. Medica (Siena). Our study and the informed consent were approved by our internal ethics committee on January 4, 2016.
Patients
The study involved 225 cycles of oocyte donation in 225 patients. We included infertile patients with characteristics and parameters justifying the need for treatment with oocyte donation.
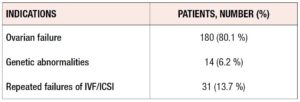
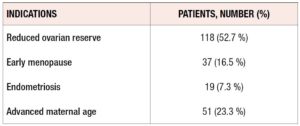
According to Italian Law, this type of treatment is open to patients without competent gametes. As seen in Table 1, the main indications were ovarian failure, repeated IVF failures, and genetic abnormalities. As shown in Table 2, “Ovarian failure” was related to multiple conditions, namely, reduced ovarian reserve, early menopause, advanced maternal age, and endometriosis.
All couples signed an appropriate consent document and a phenotype matching sheet necessary for the donor’search, as required by Italian Law, accepted by the responsible clinician of A.G.I. Medica.
Oocyte donor characteristics
The clinics where the oocytes were found are regulated by Spanish Law n. 14/2006 of May 26th on assisted human reproduction techniques and by the Royal Decree 1301/2006. Both derive from the European directives 2004/23/EC, 2006/17/EC and 2006/86/EC regarding the donation of human tissues and cells. Oocyte donation is a voluntary, altruistic and anonymous act. Choosing the most compatible donor is the responsibility of the medical team following the treatment. Every effort will be made to obtain the maximum possible immunological and phenotypic compatibility of donated oocytes with receiving patients. Receiving patients and their descendants have the right to request general information about their donor, but not the identity. The donors in this study were women aged from 18 to 28 years, resident in Spain, who underwent rigorous and comprehensive medical and psychological tests. They do not have hereditary diseases and at the time of donation they were free from infectious diseases.
According to Spanish law on assisted reproduction, six is the maximum number of children that can be born from oocytes donated by the same donor.
Oocyte donors are people of sound mind and in full possession of their mental faculties, in good health and with no family history of hereditary diseases and genetic abnormalities.
Donors underwent the following tests:
- Psychological assessment in order to exclude the existence of psychiatric, psychological disorders or inappropriate habits.
- Clinical history and medical examination to assess the donor’s personal and family history.
- Hormonal study to establish ovarian function, and a complete gynecological examination with cytology.
- Karyotype: verification of chromosomal and genetic normality. Study of the 50 mostly relevant mutations of the gene for cystic fibrosis and genetic study to rule out the fragile X mutation syndrome.
- Vaginal and cervical smear to test for Neisseria gonorrhoeae, Mycoplasma hominis, Ureaplasma urealyticum and Chlamydia trachomatis.
- Blood group and Rh factor.
- Biochemical profile, CBC and study of blood coagulation.
- Serological analytics to rule out the possibility that donors are carriers of infectious diseases
- Hepatitis B with nucleic amplification PRC (HBV-NAT)
- Hepatitis C with nucleic amplification PRC (HCV-NAT)
- AIDS with nucleic amplification PRC (HIV-NAT)
- Syphilis (TPHA-VDRL)
- IgG and IgM anti-cytomegalovirus antibodies
Recipients
The indications for oocyte donation are reported in Tables 1 and 2, while the characteristics of the recipients are reported in Table 3.
We divided the patients into two groups, according to the numbers of oocytes requested; 149 patients received a set of 3-4 and 76 patients a set of 6-8 oocytes. Each recipient received oocytes donated from a single donor only.
Endometrial preparation
Adequate hormonal preparation of the endometrium is of the utmost importance in egg donor cycles to optimize the chances of pregnancy.
The menstrual cycle is normally induced through estroprogestin pill, and, after ovarian suppression was demonstrated, the patients received estradiol valerate (Progynova ®) 6/8 mg/daily orally, depending on their serum estradiol levels. In patients with inadequate levels of estradiol or inadequate endometrial thickness, transdermal estradiol (Estraderm ®) was added. This therapy normally lasts around 14 days. Recipients were eligible for embryo transfer (ET) only when the endometrial thickness was above 6 mm. After oocyte allocation was performed, the oral estrogen was continued, together with a progesterone regime.
Oocyte warming
Oocytes were warmed using Vit Kit® Thaw, Irvine Scientific. The Thawing Solution was warmed to 37.5° C, and all other components were used at room temperature (25°). The Cryotop sample(s) to be warmed was(were) identified by the Single European Code (SEC), rapidly taken out of the liquid nitrogen, and immediately plunged into 2 ml 37°C TS solution for 1 minute. Specimen(s) were transferred to 50 µL DS solution for 4 minutes. It is necessary to gently pipette specimens once to ensure complete rinsing in DS. Then specimen(s) are transferred to the first drop of 50 µL WS solution and then to a second drop of 50 µL WS solution for 4 minutes each. Lastly, warmed oocyte(s) are transferred to pre-equilibrated culture medium, CSCM™-C (Irvine Scientific), and incubated at 37.5° C/ 6% CO2.
Oocyte insemination
Semen samples were collected by masturbation after 3-5 days of abstinence. The preparation for ICSI was performed following the World Health Organization standard protocol.
The surviving oocytes were injected with the partner’s sperm within one hour of warming using standard ICSI procedure.
Assessment of fertilization/cleavage and embryo transfer
At 16-18 hours from injection of the oocyte, fertilization was assessed by the observation of 2 pronuclei (2PN). ET was performed on day three of embryo culture, after assessment of the number of blastomeres and of the fragmentation rate. Two embryos were transferred if available and if the physical, clinical and psychological conditions of the patient and of the couple allowed it. The rest of the embryos were frozen if at the blastocyst stage or discarded if arrested.
Biochemical pregnancy was determined 14 days after ET by a positive quantitative serum beta-hCG assay > 10 IU. In the case of a positive pregnancy test, the hormonal support was continued until 12 weeks of pregnancy. Clinical pregnancy was defined as one embryo with heartbeat revealed by transvaginal ultrasonography at 5 weeks after ET.
Results
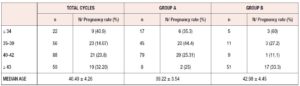
The characteristics of the patients are described in Table 3. The median female age was 40.49 ± 4 years. Of our patients, 14% were older than 43. The median male age was 42.2 ± 5.9 years.
Table 1 shows the main indications for the treatment. Of our patients, 80% were affected by ovarian failure, 13.7% by repeated IVF failures, and 6.2% by karyotype abnormalities. “Ovarian failure” was related to multiple conditions that can affect female health, such as early menopause, advanced maternal age, reduced ovarian reserve and endometriosis. We excluded patients who also received sperm donation.
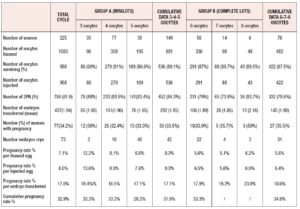
We considered six different subgroups, based on the number of oocytes obtained from Spanish banks. We then cumulated and analyzed the data of these subgroups in two major groups: Group A comprises patients (n=149) who chose a mini-lot composed of 3, 4 or 5 oocytes; Group B comprises patients who opted for a complete lot composed of 6, 7 or 8 oocytes. 1083 oocytes were thawed (601 from mini-lots and 482 from complete lots) of which 958 survived thawing.
As shown in Table 4, of the 536 inseminated oocytes belonging to Group A, 452 showed the presence of the 2PN, an indication of the occurrence of fertilization; the average number of embryos transferred was 1.95 per patient (292) and it was possible to freeze 42 supernumerary embryos. Group A recorded a pregnancy rate of 33.55%. In Group B, 482 oocytes were thawed, of which 422 were vital after thawing; 332 oocytes were fertilized. An average of 1.90 (145) embryos were transferred per patient and 31 embryos were frozen. The pregnancy rate of Group B (35.5%) was almost comparable to that of Group A (33.5%).
The pregnancy rates per thawed and injected egg were higher in Group A (respectively 8.3% and 9.3%) than in Group B (5.6% and 6.4%), but the pregnancy rates per embryo transferred were comparable in the two groups (17.1% vs 18.6%).
The results in terms of pregnancy were also compared considering the various age groups.
The median age of our patients was between 36 and 44 years as shown in Table 5. As regards pregnancy outcome, we recorded 20 miscarriages <12 weeks, 1 ectopic pregnancy, 4 late preterm births (between 32 and 37 weeks of pregnancy), and 23 term pregnancies. Five pregnancies were twin pregnancies. 29 babies were born, with a median gestational age of 37+6 weeks. Concerning the mode of delivery, 12 women had a vaginal birth and 15 cesarean section. In two cases the pregnancy was complicated by pre-eclampsia which led to cesarean section at 33 and 35 weeks of pregnancy, respectively. Another complication was postpartum hemorrhage (blood loss >1000 ml), experienced by two patients. We had one case of intrauterine fetal death >24 weeks (in a twin pregnancy), with a subsequent cesarean section at 32 weeks.
The median fetal weight was 3070 g (with a minimum weight of 2660 g and a maximum weight of 3880 g) for the singleton babies and 2265 g (with a minimum weight of 1560 g and a maximum weight of 2720 g) for twin babies. As regards the fetal sex, of the 29 babies born 16 are male, 15 are female.
Finally, we analyzed the cumulative pregnancy rates (pregnancies obtained from the fresh cycles and pregnancies obtained after transfer of thawed embryos) in the two groups. Of the 62 patients with frozen embryos, 25 patients have not yet utilized them since they achieved pregnancy with the first treatment (fresh one). Of the other 37, at present only 24 have utilized the thawed embryos (14 from Group A and 10 from Group B), obtaining 2 pregnancies in Group A and 3 in Group B. Consequently, the cumulative pregnancy rate is 31.9% for mini-lots and 34.8% for complete lots, as reported in Table 4.
Discussion
Oocyte donation is a treatment modality for a variety of complex reproductive problems. The main indications are advanced maternal age, reduced ovarian reserve, and premature ovarian failure. Due to the social impact of women entering the workforce, obtaining advanced educational degrees, and making greater use of contraceptive methods, they plan pregnancy later than a few years ago.
As is known, women are born with a finite number of oocytes. Oocytes peak at 20 weeks’ gestation, with 6–7 million, and thereafter decrease to 1–2 million at birth, 300,000– 500,000 at puberty, 25,000 by 37 years, and approximately 1000 at menopause. Alongside this quantitative decline in oocyte number, there is also a qualitative decline, primarily attributed to an increase in aneuploidy amongst aging oocytes (15, 16). A study evaluating ploidy status of over 1300 metaphase II oocytes revealed that aneuploidy rates remain relatively stable from 20 to 34 years of age, ranging between 5.2 and 10%, before increasing to 12.5–28.1% at ages 35 to 40 years, 50% at 42–43 years, and 100% at 45 years (17, 18).
Conversely, advanced maternal age does not seem to influence the thickness, pattern and receptivity of the endometrium, which with appropriate sensitization may be suitable for embryo implantation. The first references date back to early ’90s, with Piette and Sauer, who found no difference in women <40 compared with those >40 undergoing oocyte donation (19, 20).
The possibility of becoming parents is a fundamental expression of the freedom of self-determination, which concerns both family and personal matters. Accordingly, this possibility should be offered to all couples, regardless of their economic circumstances. We here describe our experience using different lots of oocytes, which represented an attempt to give all couples the opportunity to have children.
Analyzing the pregnancy rates, the difference between the mini-lots and complete lots was minimal (33% vs 35%); this was also true of the cumulative pregnancy rates (31.9% vs 34.8%), while the chance to cryopreserve supernumerary embryos was increased in the case of complete lots (approximately 28% vs 41%). In the latter case, couples may therefore have access to a second chance more often than the couples using mini-lots. However, on the basis of our experience, albeit with small numbers of patients, few couples opt for thawing of embryos as they get pregnant with a fresh treatment.
We must acknowledge that frequently the patients requesting complete lots have more economic opportunities and are often older, which explains the similar the pregnancy rate between these patients and those using minilots. Of 76 patients who requested complete lots, 51 were older than 43; conversely, only 8 out of the 149 patients requesting mini-lots were older than 43.
In this age group, the pregnancy rate was higher in patients with complete lots (33% vs 25%), suggesting that in these cases the best clinical advice may be to have a greater number of oocytes available. Possible explanations of the need for a higher number of oocytes in this age group are decreased uterine perfusion with older age, the presence of fewer E2 receptors on the endometrium cell surface, the increase in collagen content, and the reduced number of stromal cells in the endometrium, as previously described in egg donation models (21-23).
The under-34s are another age group needing extra consideration, as the patients in this group are more likely to have major clinical problems, such as previous tumors or early menopause, or genetic diseases (e.g. Turner syndrome). We only had a few patients <34 years of age and they showed pregnancy rates of 60% with complete lots and only 35% with mini-lots.
This analysis leads us to consider that general health of the patient together with her age and the cause of her infertility are elements that can indicate not only the best treatment for her, but also the most suitable number of oocytes to retrieve, in order to help her achieve a successful outcome.
Aware of the small sample size and of the numerical inhomogeneity of the two groups, we can however affirm that the laboratory results are essentially overlapping from various points of view (number of oocytes surviving, number of fertilizations, pregnancy rate per embryo transferred).
Considering also the economic aspects and our demonstration that even with a reduced number of oocytes the chances of pregnancy are good, it can be affirmed that heterologous fertilization is not a privilege for the few, but can be accessible to most couples who need it.
Conflict of interest: none.
Financial support: none.