Introduction
Vitamin is a secosteroid hormone that is produced photochemically primarily by the skin under exposure to ultraviolet-B from sunlight. It is synthesized as a fat-soluble prohormone, namely, vitamin D3 or cholecalciferol, whichan also be derived from animal products. The other form of vitamin D is dietary vitamin D2 from plant sterols, which is also called ergocalciferol [1].
A vast amount of data exists regarding the roles of vitamin D in biological mechanisms of autoimmunity, insulin resistance, cardiovascular diseases, malignancies, and in regulation of cell growth [2,3]. Vitamin D also has important effects on reproductive organs [4] and on enzyme metabolization in female reproductive tissues [5]. These tissues have been shown to regulate, metabolize, and respond to changes in endogenous vitamin D and calcium (Ca++) concentrations. It has also been suggested that vitamin D and Ca++ play roles in oocyte maturation and fertilization, endometrial receptivity, and embryo implantation [6].
However, data evaluating the effects of vitamin D on the female reproductive systems are limited, despite the presence of vitamin D receptors and of 1-alpha hydroxylase production in the ovaries, uterus, and placenta [7,8]. The primary modulating parts of the female reproductive system in early pregnancy are the Fallopian tubes, which support and regulate fertilization and delivery of the blastocyst to the endometrial cavity [9,10]. Previous studies have shown that vitamin D, and particularly calcium, play important roles in sperm motility, oocyte fertilization, and the regulation of peristalsis in the Fallopian tubes [11]. Calcium is localized in the tubal secretory and ciliated epithelial cells, and modulation has been observed in the calcium stores of both cell types during the menstrual cycle [12,13]. In addition, various Ca++ binding proteins are present in the tubal tissues and allow the regulation of tubal Ca++ contents [14].
About 1-2% of all pregnancies are ectopic, but are identified in 6-16% of pregnancies that involve bleeding and/or pain in the first trimester. The pathological implantation of blastocysts at an extra-endometrial site most frequently occurs in the Fallopian tubes, which are the primary sites for implantation in 98% of ectopic pregnancies: a series of 1800 surgically treated cases reported the distribution of the ectopic localizations as follows: ampulla 70%, isthmus 12%, fimbria 11%, ovaries 3%, interstitial 2%, and abdomen 1% [15,16]. The exact mechanism of ectopic pregnancies is unknown [17], but deteriorations in normal molecular interactions and increased receptivity of the Fallopian tubes to embryos are amonghe suggested pathophysiological mechanisms [18]. Alterations in Ca++ or vitamin D metabolism may also affect tubal motility and the transfer of the fetus to the intrauterinevity, but only limited data are available on this subject.
The present study aimed to investigate alterations of vitamin D metabolism, which adversely affect Fallopian motility, and to evaluate the correlation of serum vitamin D levels in women with typical pregnancies versus women with ectopic pregnancies.
Materials and Methods
This prospective study was conducted between October 2015 and June 2017 at the Kanuni Sultan Suleyman Training and Research Hospital Department of Obstetrics and Gynecology. The study protocol was approved by the institution’s ethics committee and registered at ClinicalTrials.gov (NCT03894735). A written informed consent was obtained from all subjects.
50 women who were diagnosed with ectopic pregnancies during the study period were included in the study group. Patients between 18 and 40 years of age, without any known operations, or chronic disorders, without any history of sexually transmitted disease(s), patients who had no miscarriages, or abnormal bleeding episodes, or deliveries in the previous 8 months, and patients with regular menstrual cycles with a minimal variation of 1-2 days during the last 8 months were included in the study. Patients who had sun exposure, used vitamin D, calcium or fatty acid supplementation, developed gestational diabetesrre-eclampsia or those who aborted were excluded from the study.
61 women who applied to the outpatient obstetrics clinic during the same period with 6-12 weeks of gestation and who met the inclusion criteria were included in the control group. These women were followed during their pregnancies and among them 53 women whose pregnancies were not complicated with any disorders and who delivered at term were included in the data evaluation of the control group.
Both intra-uterine and ectopic pregnancies were confirmed by serum beta-hCG (human chorionic gonadotropin) levels and transvaginal ultrasound examination (Voluson E8 Expert system, RAB6-D; GE Healthcare, Zipf, Austria) showing the gestational sac with a yolk sac or embryo. Gestational weeks were calculated from the first day of the last menstrual period. Blood samples were taken for biochemical analyses of serum vitamin D, calcium, magnesium and beta-hCG levels.
Total 25(OH)D is a vitamin D metabolite used in the assessment of serum vitamin D levels. However, hydrophobic 25(OH)D is usually protein bound in serum and the determination of its levels is dependent on the dissociation of 25(OH)D following interaction with the organic solvents used in the measuring kits. Therefore, a difference between measuring kits can be observed. Furthermore, conditions affecting the dissociation of 25(OH)D from its binding proteins such as pregnancy, estrogen therapy or chronic kidney disease can alter the measurements [19]. The Vitamin D3 Elecsys (Roche Diagnostics, Germany) and the Roche 9180 Electrolyte Analyzer were used to measure ionized Ca++, magnesium and 25 OH vitamin D levels.
Statistical analyses
Data analysis was performed with SPSS (version 23.0; SPSS Inc., Chicago, IL, USA). Data normality was analyzed using the Kolmogorov-Smirnov test in order to compare the quantitative data between groups. The Mann-Whitney U test was used to compare data that did not show normal distribution. Binary logistic regression analysis was used to determine independent risk factors affecting ectopic pregnancy. Statistical significance was set at a p value lower than 0.05.
Results
In the present study, data of 156 women, 50 in the ectopic (study) group and 53 in the normal pregnancy (control) group were evaluated. The mean ages of the ectopic and normal pregnancy groups were 29.34 ± 5.85 and 29.03 ± 6.58 years, respectively.
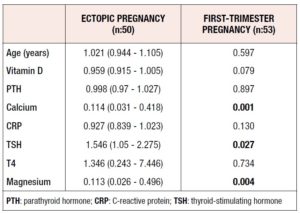
The serum levels of vitamin D were 11.09 ± 10.25 and 14.75 ± 10.14 in the ectopic and normal pregnancy groups, respectively (p = 0.006). The serum calcium levels also showed a significant difference, being 9.05 ± 0.52 in the ectopic pregnancy group and 9.78 ± 3.04 in the normal pregnancy group (p <0.001). In addition, as significant difference between the two groups in terms of serum magnesium levels were observed (2 ± 0.38 in the ectopic pregnancy group and 2.24 ± 0.29 normal pregnancy group; p = 0.001) (Table 1).
Risk factors for ectopic pregnancy were evaluated with double logistic regression analysis. The results showed that increased levels of calcium acted preventively against ectopic pregnancy. A 0.114-fold decrease in the occurrence of ectopic pregnancy was observed. The magnesium levels also displayed similar effects on ectopic pregnancy. Furthermore, an increase in (thyroid-stimulating hormone) TSH levels increased the risk of ectopic pregnancy 1.546-fold. Analysis of the rest of the variables did not yield significant results (Table 2).
Discussion
This study evaluated serum levels of vitamin D and found that women with ectopic pregnancies had significantly lower levels than women with normal first-trimester pregnancies. Sahhaf et al. found similar results in their study [20]. The serum levels of calcium were also found to be decreased in these patients, due to their lower levels of vitamin D. According to current evidence on the roles of vitamin D in the female reproductive system, deficiencies in vitamin D levels contribute to several gynecological diseases, including polycystic ovary syndrome, endometriosis, and pregnancy disorders. The presence of vitamin D receptors and mRNA in the endometrium, ovaries, and cervix suggests that vitamin D plays a role in the metabolic and pathological pathways of the female reproductive tract [21].
Even though the roles of vitamin D metabolism and calcium have been evaluated in both animal and human female reproductive systems, no study has assessed the role of vitamin D and calcium levels in ectopic pregnancies. Only one study evaluated the vitamin D-related molecules and calcium-sensing receptors in the Fallopian tubes in ectopic pregnancies, reporting that vitamin D and Ca++ may play a role in the regulation of tubal physiology and may be involved in the progression of ectopic pregnancies [9]. It has been shown that pathological changes in active, tubal vitamin D levels can cause abnormal tubal transportation of gametes or implantation of embryos. In addition, in this study, all the proteins (VD synthesizing (CYP27B1) and catalysing (CYP24A1) enzymes, VD-binding protein (VDBP), VD receptor (VDR), retinoid X receptor (RXR) and CaSR) examined by immunohistochemistry at the implantation sites of the patients with ectopic pregnancies were significantly higherhere than in the more remote areas of the tube where the ectopic focus was located [9].
Moreover, the authorsund that the tubal samples from ectopic pregnancies had significantly altered vitamin D-related molecules and calcium-sensingceptors, representing pathological alterations in tubal vitamin-D levels and related genomic signaling pathways that eventually result in abnormal tubal transportation or implantation of gametes or embryos in the Fallopian tubes [21]. Combined with our findings of decreased levels of vitamin D and calcium in tubal pregnancies, this research makes it apparent that both vitamin D and calcium metabolism have a systemic involvement in the etiology of the ectopic implantation of the embryo. In our study, low magnesium and calcium levels are thought to lead to ectopic pregnancy.
Nevertheless, several other explanatory mechanisms cannot be excluded when evaluating the pathophysiological factors associated with ectopic pregnancies, including the immune-modulatory and anti-inflammatory characteristics of vitamin D [22-24]. Inflammation may play a significant role in inhibiting ciliary movement in the Fallopian tubes, which causes ectopic implantation of the gamete. Inflammatory molecules, including prostaglandins, cytokines, etc., may cause premature implantation [24]. Another study found that low serum vitamin D levels were associated with preterm delivery [25,26]. The present study showed decreased levels of vitamin D and calcium, which may cause inflammation of the tubal epithelium, which, in turn, eventually inhibits ciliary motility. Similarly, in another study, serum vitamin D intake during pregnancy has shown to affect ciliary activity in the respiratory system, which in return affects wheezing symptoms [27]. In our study, no significant difference in CRP (c-reactive protein) values was observed between the two groups.
The limitations of the present study are, first, the relatively small sample size, even though it was comparable with those of other previous published studies. Second, the vitamin D differences between ectopic and intrauterine pregnancies may have been affected by the time of the year when the sampling was done. Third, it is known that the measurement of serum 25 (OH) D levels varies between kits, especially in pregnant women [21,22]. One of the strengths of the study was the strict exclusion criteria which resulted in a cohort of women without pre-eclampsia and gestational diabetes mellitus, which are known to lower vitamin D levels according to previous studies.
Conclusion
The serum levels of vitamin D, magnesium and calcium were lower in patients with ectopic pregnancies compared to patients with normal first-trimester intrauterineregnancies. These findings might be explained by alterations in tubal ciliary motility as a result of calcium deficiency. These results should be supported by clinical studies in larger cohorts, and by preclinical studies, which might shed light on the possible underlying mechanisms.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Kanuni Sultan Suleyman Training and Research Hospital (2017) and registered to ClinicalTrials.gov (NCT03894735).
Informed Consent: All the patients gave written consent and permission for the use of data in studies.
Peer-review: Externally and internally peer-reviewed.
Authorship Contributions Treatment: P.Y.B. Concept: P.Y.B and N.F.T.S. Design: G.T Data Collection or Processing: P.K. Analysis or Interpretation: P.K and K.Ç. Literature Search: P.Y.B. and N.F.T.S. Writing: P.Y.B. and G.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.