Introduction
Primary hypothalamic pituitary amenorrhea is an amenorrhea caused by hypothalamic or pituitary dysfunction affecting the function of the hypothalamic-pituitary-ovarian axis. A common cause is congenital hypogonadotropic hypogonadism (CHH). It is a genetically heterogeneous disorder in which a congenital impairment of gonadotropin-releasing hormone (GnRH) neuronal function leads to impaired synthesis and secretion of GnRH in the hypothalamus, which in turn leads to reduced gonadotropin secretion in the pituitary gland, thus resulting in gonadal insufficiency [1,2]. Congenital hypogonadotropic hypogonadism (CHH) is divided into Kallmann's syndrome with reduced or impaired olfaction and idiopathic hypogonadotropic hypogonadism (IHH) with normal olfaction (normosmic IHH, nIHH) [3-5]. Kallmann's syndrome accounts for approximately 40-60% of all CHH cases [6,7]. The incidence of CHH is greater in men than in women (approximately 1:10,000 men and 1:50,000 women) [6]. Relatively few cases of CHH have been reported in women, who usually present with primary amenorrhea and delayed puberty [8,9]. CHH has negative effects on patients' reproductive function, bone and metabolic health and mental health [10-12]. Therefore, early diagnosis and timely treatment of CHH is very important. We report a case of a woman with primary hypothalamic-pituitary amenorrhea who successfully delivered a healthy infant after hormone replacement therapy (HRT) and then pulsatile GnRH.
Case report
The patient is a 25-year-old woman who came to our clinic on July 25th, 2014, complaining of no menarche and poorly developed breasts and vulva. Upon physical examination her height was 176 cm, weight 58.5 kg, and body mass index (BMI) 18.8 kg/m². She presented with hyposmia. Both breasts were palpable outside the areola with no development of the areola (Tanner Stage 2 for breast [13]). She had no pubic hair and the labia minora were not fully developed.
Investigation
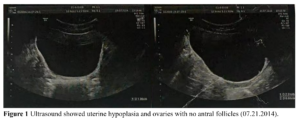
Ultrasound examination of pelvic organs showed an immature uterus with a volume of 3.31 cm³ (2.3 х 1.6 х 0.9 cm). The endometrium was thin and linear. The right ovary had a diameter of 2.0 cm and the left ovary a diameter of 1.9 cm. No obvious antral follicles were found in both ovaries (Figure 1). Serum sex hormones were follicle stimulating hormone (FSH) 2.02 IU/L, luteinizing hormone (LH) 0.45 IU/L, estradiol (E2) 18.08 pg/mL, progesterone (P) 0.07 ng/mL, prolactin (PRL) 5.8 ng/mL, and testosterone (T) 8.57 ng/mL. MRI of the pituitary gland suggested it was slightly smaller. Clinical diagnosis was CHH, Kallmann’s syndrome.
Treatment
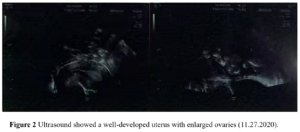
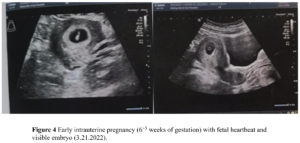
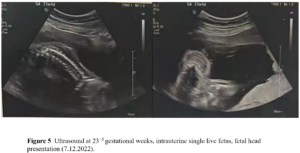
The patient had no menarche yet and desired to have a period. She was treated with 17β-estradiol 2 mg/day for 14 days which was combined with sequential dydrogesterone 10 mg/day for next 14 days/cycle. After 19 cycles of treatment there was uterine bleeding. After that, her menstruation was regular during the use of HRT. After 6 years of HRT treatment, she got married and had the desire to conceive. At this moment her uterine size was normal (Figure 2) and her serum sex hormones were FSH 1.76 IU/L, LH 0.33 IU/L, E2 21.21 pg/mL, P 0.15 ng/mL, PRL 7.4 ng/mL, and T 12.86 ng/mL (Figure 3A and Figure 3B). From June 2020 to October 2020, the patient was treated with once-a-day oral letrozole 5 mg from day 3-7 after menstruation initiation and once a day subcutaneous human menopausal gonadotrophin (hMG) 150 IU for 5 days. This scheme was given for 5 cycles but there were no mature follicles. Considering that the patient did not develop dominant follicles with hMG ovulation promotion therapy, in November 2020, she was treated with pulsatile GnRH 10 µg/90 min gonadorelin given through pump. On the 16th day of the cycle, her serum sex hormones were FSH 2.59 IU/L, LH 5.42 IU/L, E2 204.89 pg/mL, P 0.4 ng/mL, PRL 12.67 ng/mL, T 19.71 ng/mL and the transvaginal ultrasound showed the largest follicle on the left side (1.93 х 1.1cm). Patient had dominant follicles on three consecutive cycles but she could not become pregnant. Patient stopped her medication from November 2020 except pulsatile GnRH. Patient visited our clinic on march 7, 2022 with a delay of 10 days in her menstruation. On that day her test results were: β-hCG 2663,53 IU/L, P 26.76 ng/mL, PRL 11.73 ng/mL, E2 137.68 pg/mL. She was then given dydrogesterone for luteal support and pulsatile GnRH pump was withdrawn. On March 21, 2022 her test results were β-hCG 47,435.57 IU/L, P 24.08 ng/mL, E2 194.56 pg/mL (Figure 3C and Figure 3D). Transabdominal pelvic ultrasound showed the uterus in anterior position, measuring 6.1 х 5.3 х 4.7 cm, with a homogenous echo muscular layer and an embryo seen in the uterine cavity. The size of the gestational sac was about 2.3 х 2.3 х 2.0 cm, with embryonic cardiac pulsation was visible, the long diameter of the embryo was about 0.8 cm. Ultrasound hints: early live intrauterine pregnancy of 6+5 weeks of gestational age (Figure 4). Dydrogesterone luteal support therapy was continued up to 12 weeks. Ultrasound screening showed a normal fetus of 23+5 weeks of gestation (Figure 5). The rest of the pregnancy developed uneventfully and a caesarean section, due to failed labor induction, was performed at 40+4 weeks of gestation, of which a healthy masculine infant weighing 3,560 g was delivered.
Discussion
A common cause of primary hypothalamic pituitary amenorrhea is CHH. The pathogenesis of CHH is a defect in the development of the hypothalamus or pituitary gland, leading to impaired secretion or reduced synthesis of GnRH, hence resulting in a selective deficiency of gonadotropins and a chronic lack of sex hormones. The latter leads to failed genital development, and a lack of sex hormones which also produce endocrine and metabolic abnormalities, such as hypertension, diabetes mellitus, and the metabolic syndrome. These conditions can increase long-term risks: cardiovascular disease risk, osteoporosis and fractures [14].
Sequential HRT if the first-line treatment for CHH in order to induce puberty and the development of secondary sexual characteristics and normal menstrual cycles [9]. The goals of CHH treatment are (1) promote the development of secondary sexual characteristics; restore normal sexual function, improve libido, and enhance the quality of sexual life; (2) improve bone density and prevent osteoporosis; (3) reduce the risk of cardiovascular events; and (4) promote fertility [15].
Our case came to us at age 25 without having presented menarche and having a uterine hypoplasia. She was diagnosed with CHH. She started HRT since 2014 in order to develop the uterus and maintain secondary sexual characteristics. After 19 months of HRT, she had her first withdrawal bleeding, and since then regular monthly menstruations occurred. During HRT use, we performed pelvic ultrasonography every year to assess uterine size and endometrial thickness, observing a gradual increase in uterine size, reflecting the efficacy of HRT.
When she became 32, she married and desired to become pregnant. After one month of using pulsatile GnRH, the patient had her first spontaneous ovulation and after 17 months she became pregnant and subsequently gave birth to a healthy infant.
Pulsatile GnRH therapy is an option for women of reproductive age with CHH. Pulsatile GnRH pump therapy mimics the physiological hypothalamic pulsatile release of GnRH needed to induce ovulation in patients with CHH. Pulsatile GnRH therapy restores cyclic release of FSH and LH from the pituitary gland, thus, promoting gonadal maturation and secretion of sex steroid hormones: estrogen, progesterone and androgens [16]; and in addition to restoring spontaneous ovulation in CHH patients and regain their fertility.
Conclusion
In patients with primary hypothalamic pituitary amenorrhea and uterine hypoplasia, sequential hormone therapy should be used first to promote uterine development, followed by a pulsatile GnRH treatment to successfully induce ovulation, have a spontaneous pregnancy and subsequent live birth.
Funding: This work was supported by the first batch of Beijing maternal and child health specialist demonstration units ‘menopausal health specialist’ [(2017)35] and the Beijing Municipal Health Commission and Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20181401) provided to Xiangyan Ruan.
Informed consent statement: Not applicable.
Acknowledgments: Not applicable.
Conflicts of interest: The authors declare no conflicts of interest.