Introduction
Endometriosis is frequently seen in women during their reproductive age and generally leads to dysmenorrhea, dyspareunia, menstrual irregularity and infertility. This condition is defined as the presence of endometrium out of the uterine cavity [1]. Although extrapelvic endometriosis is not common, it may affect many organs such as the lung, appendix, nose, umbilicus, peritoneum, intestinal wall and brain [2]. Extrapelvic endometriosis most commonly appears as cutaneous endometriosis and generally develops at the obstetric or gynecological surgical scars.
The incidence of surgical scar endometriosis, which develops after surgery (cesarean surgery and laparoscopic endometriosis surgery) may range between 0.03-0.4% [3,4]. Incision-site endometriosis is mostly observed between subcutaneous tissue and the fascia while it may be also rarely seen between the fascia and muscle or muscle and parietal peritoneum.
The symptoms of scar endometriosis are characterized by non-specific cyclic pain and palpable mass [5]. It may present as a palpable swelling at any site along the incision that grows during the menstrual period, with continuous pain on the swelling site and exacerbation of this pain during the menstrual period. It can also present as only pain during the menses.
Fibrotic tissue, suture granuloma, incisional hernia, lipoma, abscess, cyst or a foreign body should be considered in the differential diagnosis. The diagnosis is not easy since it can be misdiagnosed with these conditions [6]. The preoperative preparation of the patients, who have palpable mass on the abdominal wall and whose complaints of swelling and pain have a cyclic pattern, should involve transabdominal ultrasonography of the superficial tissue and image-guided fine needle aspiration biopsy [7-9].
Surgical excision is the treatment method with proven efficacy and the highest success rate for incisional endometriosis [3]. However, some patients may refuse surgical treatment because of various reasons and may prefer to receive medical treatments such as consecutive oral contraceptives (continuous treatment for 21 pills) or other pharmacological agents. It is known that dienogest, which is used in the treatment of endometrioma, can also be used to reduce the symptoms in the patients who refuse the surgical treatment or specifically demand medical treatment.
There are many studies regarding the use of dienogest for the treatment of endometrioma in the literature whereas there is insufficient data on medical treatment of cesarean-associated incisional endometriosis. Based on this, as the first study in literature, we aimed to evaluate the efficacy of surgical and medical treatments (dienogest) for cesarean-associated abdominal wall endometriosis.
Methods
In the present study, patients with a history of previous cesarean section who applied to our clinic between 1st March 2007 and 1st January 2017 with the complaints of hardness in the incision line, palpable mass and pain accompanied with hardness during menstruation, enlarging palpable mass and increasing pain were evaluated. The pain was assessed by Visual Analogue Scale (VAS) and the scores were recorded. These patients were prediagnosed with endometriosis by symptomatic and ultrasonographic evaluation. GE Voluson S6 machine with C1-5-RS H40462LA and 12L-RS H40402LY probes were used for ultrasonographic examination. Then, fine needle aspiration biopsy was performed under local anesthesia for pathological confirmation and definite diagnosis [7-9]. The study included 45 patients, who were diagnosed with endometriosis according to biopsy results. The date of the latest cesarean section was recorded to determine the duration of scar endometriosis development after cesarean section. Patients were informed that the definitive treatment of incisional endometriosis is surgery. They were also informed about other medical treatments and dienogest. Of the 45 patients diagnosed with incisional endometriosis, 23 patients preferred surgical treatment option. Pain status of the patients was recorded preoperatively and postoperatively. Dienogest was administered in the other 22 patients who refused the surgical treatment. Preoperative 3-dimensional measurement of the endometriotic foci was performed and mean values were calculated. These patients were followed monthly by measuring endometriotic foci during the 1-year period. Dienogest treatment was stopped after one year, but the patients were continued to be followed-up for the next 3 years by performing endometriotic foci evaluation and determining the pain status in each year.
Written informed consents were obtained from the patients. Ethical approval for the study was obtained from the local ethics committee and the study was carried out according to 2008 Helsinki Declaration.
Data analysis of the study was performed using SPSS software package version 22.0 for Windows. Values are presented as mean ± standard deviation (SD). Analysis of data was performed by means of non-parametric statistical methods (Mann-Whitney U test, Wilcoxon test, Bonferroni Post Hoc test, Spearman correlation test, linear regression analysis and the Friedman test) since the numbers of the patients in the surgical and medical treatment groups were less than 30 subjects. A value p< 0.05 was considered as statistically significant.
Results
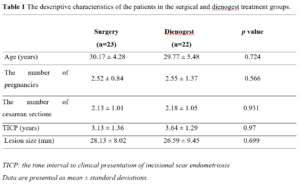
The information about the descriptive characteristics of the patients in the surgical and dienogest treatment groups are presented in Table 1. No statistically significant differences were found between both groups in terms of mean age, the number of pregnancies, the number of cesarean sections, the time interval to clinical presentation of incisional scar endometriosis (TICP) and also the lesion size (Table 1).
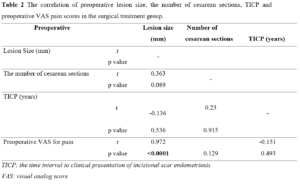
As a result of linear regression analyzes and other correlation tests, no significant correlation was found between the number of preoperative cesarean sections and preoperative VAS pain scores, and between TICP and preoperative VAS pain scores in the surgical treatment group (p=0.089 and p=0.536) whereas preoperative lesion size was found to be significantly correlated with preoperative VAS pain scores (r=0.972; p= <0.0001) (Table 2).
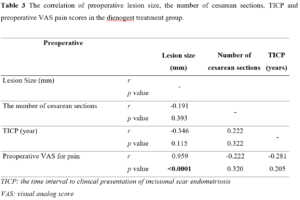
On the other hand, pretreatment lesion size and VAS pain scores were found significantly correlated in the dienogest group. These results indicate that there is a correlation between pretreatment lesion size and pretreatment VAS pain scores (Table 3 and Table 4). Based on these findings, it may be suggested that in scar endometriosis pain increases as lesion size becomes larger (Table 3 and Table 4).
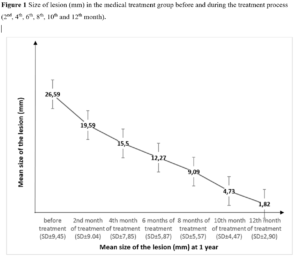
A statistically significant difference was found in the size of lesions in the medical treatment group measured at the beginning and at the 2nd, 4th, 6th, 8th, 10th and 12th month of treatment (c2= 125.429; p<0.0001). This indicated that the results obtained from the measurements of lesion sizes were not similar. Therefore, Bonferroni Post Hoc test was applied to determine the origin of difference between the measurements. This revealed that lesion sizes progressively decreased by repeated measurements after a certain elapsed time (Figure 1).
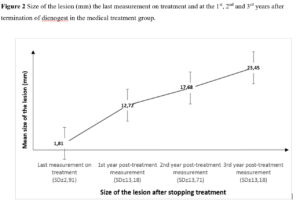
A statistically significant difference was observed among the lesion sizes at the 1st, 2nd and 3rd year after medical treatment was stopped in the patients (c2= 16.545; p<0.0001). This indicated that the results obtained from the measurements of lesion sizes were not similar. Therefore, Bonferroni Post Hoc test was applied to determine which measurements showed difference. Mean lesion sizes progressively decreased by repeated measurements after a certain elapsed time (Figure 2).
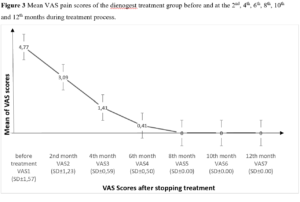
On the other hand, the difference between mean preoperative VAS pain score (4.61±1.305) and mean postoperative VAS pain score (0.00± 0.00) was statistically significant in the surgical treatment group (p<0.0001). Also, the difference between mean pretreatment VAS pain score and mean VAS pain scores at the 2nd, 4th, 6th, 8th, 10th and 12th month of treatment was found statistically significant in the medical treatment group (c2= 126.818; p<0.0001) (Figure 3). Mean pretreatment and mean post treatment VAS pain scores (2nd month) were 4.77±1.572 and 3.09±1.231 in the dienogest treatment group, respectively. The difference between these values was statistically significant in favor of post -treatment period (p< 0.0001). In conclusion, pain was statistically significantly decreased in the patients treated with dienogest (Figure 3).
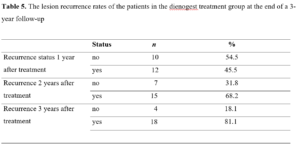
The lesion recurrence status of the medical treatment group during 1st, 2nd and 3rd year of follow-up after treatment is presented in Table 5. No recurrence occurred during the first year of follow-up in 54.5% of the patients whereas 45.5% patients developed recurrence. One year after cessation of treatment, cyclic pain relapsed only in patients with lesion recurrence. There was no pain complaint in patients without lesion recurrence. During the second year of follow-up, the rate of lesion non-recurrence was 31.8% while the recurrence rate was 68.2%. At the third year, no lesion recurrence was found in 18.1% of the remaining patients, whereas 81.8% patients developed recurrence. Among the patients who received medical treatment; 81.8% were surgically treated at the end of three years. In addition, during this three-year follow-up, 18.1% of the patients, who were treated with dienogest, had no recurrence and did not need surgical treatment (Table 5). Similar to the recurrence of pain at the end of one-year, cyclic pain relapsed only in patients with lesion recurrence at the end of the second and third year of follow-up. Patients without lesion recurrence did not have pain complaint.
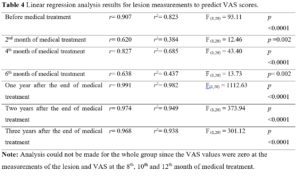
A regression analysis was performed between the VAS score, which is an indicator of the presence and severity of clinical pain, and patient age, number of pregnancies, number of previous cesarean sections, time of occurrence of the lesion, and lesion size. A statistical relationship was found only between VAS score and lesion size (Table 4). No other statistical relationship was found. Linear Regression analysis was applied to understand the predictive power of lesion measurements on VAS score (Table 4).
The lesion measurements of the patients in different time periods gave significant correlations with VAS scores. It was seen that lesion size (as a variable) in different time periods explains the lowest 38% and the highest 98% of the total variance of VAS scores. By examining t-test results, regarding the significance of the regression coefficients, it was observed that the lesion variables are significant predictors of the VAS scores, regardless of the time period.
In addition, patients who preferred surgical treatment were followed-up for 3 years in the postoperative period. None of them showed recurrence during follow-up The treatment success rate for the surgically treated patients was recorded as 100%.
Discussion
It is known that subcutaneous endometriosis that develops in the incisional scar tissue results from the inoculation of endometrial cells. This theory is confirmed by the formation of subcutaneous endometriosis in the abdominal wall through carriage of endometrial tissue during the menstrual cycle [10]. The frequency of incisional scar endometriosis is associated with surgery. The development rate of scar endometriosis after hysterotomy performed in second trimester abortion is 5.08% whereas its frequency ranges between 0.03-0.4% after cesarean section [4].
Endometriotic mass has similar characteristics to deep pelvic endometriosis upon ultrasonographic examination, which is performed to support clinical evaluation. A hyperechogenic ring is commonly seen in ultrasonographic images which is caused by the hypoechogenic nodule with indistinguishable contours and surrounding inflamed adipose tissue [7].
Among the 45 patients of our study, we encountered heterogeneous formations with indistinguishable contours and various sizes. Although, it can be easily diagnosed based on evidence of the painful nodule in the incision site after the cesarean section or gynecological operation in the obstetric clinics, it is generally found to be inaccurately prediagnosed in other departments except for the gynecology and obstetrical department [11,12].
The presence of cyclic pain in a mass detected in the cesarean scar should be evaluated pathologically with respect to scar endometriosis. Imaging techniques are not specific. The definite diagnosis of incisional scar endometriosis is based on pathological examination. The preoperative preparation involving transabdominal ultrasonography and image-guided fine needle aspiration biopsy can be an alternative to surgery for the diagnosis in patients with prediagnosis of incisional scar endometriosis and palpable mass in the abdominal wall [7-9,13-15]. The definite diagnosis should be made by fine needle aspiration biopsy to rule out malignancy and other potential pathologies in the patients who refuse surgery [7-9,13-15]. However, fine needle aspiration biopsy is not recommended in the cases with suspected incisional hernia because of the risk of intestinal injury [16]. In addition, we confirmed the diagnosis by performing fine needle aspiration biopsy before the treatment.
The time interval to clinical presentation of incisional scar endometriosis may vary between 45 days to 20 years [6,17]. Horton et al. [6] have evaluated the data of 445 cases of abdominal wall endometriosis and found that this time interval can be 3.6 years on average. In our study, the earliest detected case emerged 24 months after the operation while the latest case was detected 84 months after. Mean clinical presentation time was found to be 3.55 years and this result was consistent with the literature.
It has been reported in the literature that the diameter of the nodules can range from millimeters to 6 cm in scar endometriosis [6,18]. Similar results were obtained in our study. The diameters of the smallest and the largest detected endometriosis were 7 mm and 48 mm, respectively.
Surgical therapy is the primary option in the treatment of endometriosis that develops in the surgical incision scar after the operation [3,19,20]. The recurrence rate is very low after total resection [3]. Similarly, in our study, no recurrence was seen in short- and long-term follow-ups in the patients who preferred surgical treatment option and this finding is also consistent with the literature [21].
On the other hand, medical treatment can be administered to the patients who refuse surgical treatment. It has been reported that nonsteroidal anti-inflammatory agents, oral contraceptives, gonadotropin releasing hormone (GnRH) analogues and progesterone can be used for the medical treatment to decrease the diameter of the endometriotic lesion, to facilitate surgery and to reduce symptoms. However, it has been determined that medical treatment provides a temporary improvement of symptoms and that these recur if medical treatment is stopped. Therefore, efficacy of medical treatment is low [6,22,23].
There are reports in which dienogest has been used to treat for 6 and 12-months patients with endometrioma. Dienogest decreased mean endometrioma diameters and related pain, dysmenorrhea and dyspareunia [24-26]. It has been additionally reported that 12-month dienogest treatment significantly reduced pain, dysmenorrhea, and dyspareunia in the patients with symptomatic endometriosis [27,28]. Dienogest treatment has been implemented to prevent recurrence in patients operated for ovarian endometrioma and its efficacy in prevention of recurrence found to be statistically significant [29].
Dienogest treatment for incisional scar endometriosis after confirming the diagnosis by fine needle aspiration biopsy may be a secondary treatment option for patients who prefer avoiding surgery. In our study, 4 (18.1%) patients were recovered after receiving dienogest treatment without the need for surgery.
In conclusion, definite diagnosis of a mass detected in the cesarean section scar can be established only by biopsy. A wide local excision seems to be the best treatment choice to prevent recurrent lesions. However, dienogest treatment, administered after confirming the diagnosis by fine needle aspiration biopsy, may be a secondary treatment option to avoid surgery until absolute medical necessity and save time until planning pregnancy. It can offer a single operation solution to patients who will give birth by cesarian section. Thus, there will be no need for a second surgery.