Introduction
SERMs (selective estrogen receptor modulators) are synthetic molecules that bind to the estrogen receptor and produce agonistic activity in some tissues while being estrogen antagonist in other tissues. For instance, all SERMs, whether in practice or those that never made it to the market, are estrogenic in the bone and the venous system while some are estrogenic in the vagina and the uterus. Other SERMs are neutral in vagina and uterus. Virtually all SERMs are estrogen antagonists in the breast, and some are neutral or even antagonistic in the uterus. Thus, unlike estrogens, which in the United States have a phenomenon known as “class labeling” by the Food and Drug Administration (FDA), not all SERMs have equal effects in all tissues.
This review will concentrate on current SERMs in clinical practice, which include tamoxifen, raloxifene, bazedoxifene (alone or combined with conjugated estrogen then known as a tissue specific estrogen complex) and ospemifene.
Tamoxifen
Tamoxifen is the most widely prescribed antineoplastic drug worldwide. It is used as adjuvant therapy for patients with estrogen receptor positive breast cancer. In this review, we will only address its use in breast cancer chemo prevention.
The rationale for tamoxifen and breast cancer chemoprevention stems from its inhibition of mammary tumors in mice and rats as well as suppressing the MCF-7 cell line, an estrogen sensitive breast cancer cell line in vitro. In addition, various treatment studies have shown a reduction in contralateral new onset breast cancer. Thus, in 1992, a breast cancer prevention trial (BCPT) was launched [1]. Thirteen thousand three hundred and eighty-eight women aged 35 or older were randomized to placebo versus tamoxifen 20 mg per day. Their breast cancer risk in the next five years was deemed to be greater than 1.66% using the Gail Model. The average follow-up was 44 months. The trial was stopped early in April 1998 for ethical reasons. At that point, the incidence of invasive breast cancer in tamoxifen patients was 3.4 cases/1,000 women years versus placebo 6.8 cases/1,000 women years. This represented a 49% reduction (p < 0.00001). The data safety monitoring board felt it was unethical to allow one half of these high-risk patients to continue to take placebo. In October 1998, in the United States, the FDA approved tamoxifen for primary prevention of breast cancer in women at high risk. The FDA recommended that its use be limited to high-risk women because of potentially serious side effects. Those included thromboembolic events in terms of deep vein thrombosis, pulmonary emboli, and retinal vein thrombosis. In addition, there was an increase in endometrial carcinoma, endometrial hyperplasia, endometrial polyps, and possibly even sarcoma.
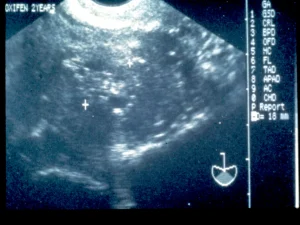
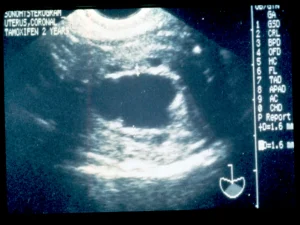
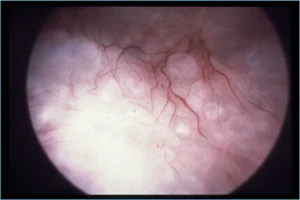
Specifically looking at tamoxifen’s effects on endometrial cancer, in the mid- to late 1980’s, a series of “Letters to the Editor” and case reports suggested an association between tamoxifen and endometrial neoplasia [2]. In 1994, there was the first publication outlining an unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen, in which some patients were displaying bizarre, heterogeneous echo patterns centrally located in the uterus which represented a loss of the normal junctional zone [3]. This was being misinterpreted as “endometrial thickening.” When viewed with saline infusion sonohysterography (SIS), these changes were often microcystic changes which represented dilated cystic atrophic glands and were located in the endometrium, the proximal myometrium, or even within polyps (Figure 1 and Figure 2). When viewed hysteroscopically, the surface epithelium is pale and atrophic, and shows coarse vessels typical of atrophy as well as blebs of these microcystic, dilated glands underneath the surface epithelium (Figure 3). Work by Berlière et al. [4], indicated that there appeared to be two distinct groups of women for developing pathology of the endometrium while on tamoxifen therapy. In that study, 575 patients with breast cancer were studied up to 5 years. All women initially had transvaginal ultrasound and then hysteroscopy, if the endometrium was not thin, compatible with atrophy. 16.6% had endometrial polyps prior to tamoxifen therapy. In the group with no initial polyps, 12.9% developed benign polyps through the observation period, but only 0.7% developed atypical hyperplasia. In the group with an initial polyp that was removed, 17.6% developed polyps and 11.7% developed atypical hyperplasia. Thus, the at-risk group had 18 times the incidence of atypical hyperplasia when followed through 5 years. Such systematic pretreatment screening can identify a high-risk group of patients who may require ongoing surveillance. In 2006, the American College of Obstetricians and Gynecologists (ACOG) in their Committee Opinion number 336 [5], which has been reaffirmed in 2014 and 2020, stated, “emerging evidence suggests the presence of high- and low-risk groups based on the presence or absence of benign endometrial polyps before tamoxifen therapy. Thus, there may be a role for pretreatment screening of postmenopausal women with transvaginal ultrasound and sonohysterography, when needed, before initiation of tamoxifen therapy.”
There are some special considerations for younger, premenopausal women. Tamoxifen has been approved for both pre- and postmenopausal women. Overall, in the Breast Cancer Prevention Trial [1] the relative risk (RR) of endometrial cancer in tamoxifen-treated patients was 2.4 and was statistically significant and the overall risk of deep venous thrombosis/pulmonary embolism (DVT/PE) was 2.5 and was statistically significant. However, when the patients were divided into less than 50 and greater than 50 years of age, the closest thing we would have today to pre- and postmenopausal, neither endometrial cancer nor thrombogenicity were statistically significantly increased in the premenopausal women. Thus, concerns that serious adverse events with tamoxifen chemoprevention may diminish the potential benefit do not seem as powerful in those women less than 50 years of age. This has significant clinical consequences for healthcare providers who do take care of such premenopausal patients.
Raloxifene
Raloxifene is a benzothiophene SERM versus the triphenylethylene tamoxifen. It has SERM-like properties as well, being an estrogen agonist in bone remodeling and lipid metabolism. In December 1997, it was FDA approved for the prevention of osteoporosis, and this indication was extended to treating osteoporosis in 1999. This was based on data from the MORE Trial [6]. That trial involved 7,700 women who were postmenopausal with osteoporosis and followed through 48 months. There was a statistically significant improvement in bone mineral density, both at the lumbar spine and femoral neck relative to placebo (p = 0.001 versus placebo and baseline). In terms of fracture reduction, the group was divided into patients without preexisting vertebral fracture and those with preexisting vertebral fracture. In the group with no preexisting vertebral fracture, there was a 49% reduction compared to placebo (95% CI = 0.35-0.73), and in the higher-risk group of patients with existing vertebral fracture, the reduction of subsequent vertebral fracture was 34% (95% CI = 0.55-0.81).
In the MORE Trial, invasive breast cancer cases were captured as an adverse event [7]. In the raloxifene group, there were 22 cases of invasive cancer for a rate of 1.3/1,000 women years versus 39 cases in the placebo group for a rate of 4.7/1,000 women years. Thus, the RR was 0.28 (95% CI = 0.17-0.46).
Raloxifene did not exhibit tamoxifen-like effects on the endometrium [6]. There was no difference from placebo on endometrial thickness on transvaginal ultrasound, endoluminal masses on sonohysterography, or proliferation or hyperplasia on biopsy. Overall, in the MORE Trial [6], there was a 20% reduction in endometrial cancer compared to placebo (RR 0.8, CI = 0.21-2.67), which was not statistically significant. This is compared to the 4-fold increase in endometrial cancer with tamoxifen in the BCPT for those women over 50 [1].
A subsequent study known as STAR (Study of Tamoxifen and Raloxifene) was a breast cancer prevention study. Because it would be unethical to have a placebo group among such high-risk women, it compared raloxifene 60 mg per day with tamoxifen 20 mg per day in women at high risk of breast cancer [8]. Through 4 years, raloxifene was as effective as tamoxifen in the prevention of primary invasive breast cancer. Initially, it was less effective than tamoxifen in the prevention of non-invasive breast cancer (lobular carcinoma in situ [LCIS] and ductal carcinoma in situ [DCIS]), although still better than an “idealized” placebo group. However, with continued follow up through 7 years, raloxifene was just as effective as tamoxifen in reducing the incidence of DCIS and LCIS [9]. In addition, compared to tamoxifen, raloxifene resulted in fewer thromboembolic events, fewer endometrial cancers, and fewer cataracts.
Bazedoxifene
Bazedoxifene is yet another SERM that exists in some countries as a “stand alone” drug for the prevention and treatment of osteoporosis. A meta-analysis of four randomized placebo control trials [10] showed that bazedoxifene can significantly decrease the incidence of vertebral fracture at follow up of 5 to 7 years. It also demonstrated long-term favorable safety and tolerability of bazedoxifene with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, and breast carcinoma. It did, however, result in an increase in hot flashes and leg cramps which are well known SERM class effects.
In another 6-month placebo-controlled trial of healthy postmenopausal women [11], bazedoxifene showed no stimulation of the endometrium, and in higher doses, actually resulted in a decrease in endometrial thickness on transvaginal ultrasound as well as bleeding when compared to placebo (p = 0.001). Thus far, it has been the only SERM to suggest endometrial suppression.
Tissue specific estrogen complex (TSEC)
In trying to identify the ideal menopausal therapy, one would look for an agent that had positive effects on the skeleton in terms of increased bone mineral density and decreased fracture, positive effects on lipids in terms of decreasing total and LDL cholesterol, positive effects in the central nervous system in decreasing vasomotor episodes without negative effects in the reproductive system (decreasing or neutral uterine stimulation, no more vaginal bleeding or spotting than placebo, improvement in vaginal dryness, irritation or dyspareunia), no increase in the risk of cardiovascular disease, and no increase in breast pain, breast density, and, ideally, even a decrease in breast cancer risk. The concept of a tissue selective estrogen complex (TSEC) is partnering a SERM with one or more estrogens to achieve pharmacologic results based on their blended tissue selective activity [12]. There really is no such thing as estrogen; there are estrogens. The Women’s Health Initiative (WHI) utilized conjugated equine estrogen (CEE) in women without a uterus and conjugated equine estrogen (CEE) with medroxyprogesterone acetate (MPA) in women with a uterus. In trying to choose the ideal estrogen to construct and optimize a TSEC, consider the following:
- Fournier et al. [13] found estradiol alone in hysterectomized women had a 30% increase in invasive breast cancer.
- In the Million Women Study which looked at 1.08 million women aged 50-64 years, the incidence of invasive breast cancer was studied with estradiol alone and estradiol combined with any progestogen [14]. Oral estradiol compared to placebo had a RR of 1.32 (p < 0.0001), transdermal estradiol RR compared to placebo was 1.24 (p < 0.0001), estradiol implants compared to placebo RR equaled 1.65 (p < 0.0001). These risks were tripled with the addition of any progestogen. There have been some criticisms of this data from the Million Women Study mainly in terms of potential biases, statistics, and internal and external consistency. Shapiro et al. [15] concluded that “Hormone replacement therapy may or may not increase the risk of breast cancer, but the Million Women Study does not establish that it does.” Regardless of whether there is an increase or not, there is no suggestion of a decrease in breast cancer.
- When the WHI was looked at after 11.7 years [16], the group that received CEE/MPA had a 25% increase in invasive breast cancer that was statistically significant (95% CI 1.07-1.46) whereas those with a hysterectomy who received CEE alone had a 23% reduction in invasive breast cancer (95% CI 0.62-0.95). Thus, based on all this it becomes obvious that the addition of a progestational agent to any of the estrogens increases the risk of invasive breast cancer, but CEE alone actually reduced the risk of invasive breast cancer whereas estradiol has never been shown by itself to do that. Furthermore, Manson et al. [17] publish an 18-year follow up to the WHI which showed no increase in all-cause mortality, cardiovascular mortality, or cancer mortality whether patients received CEE/MPA or CEE alone. However, specifically in looking at breast cancer mortality, those who received CEE+MPA had a 44% increase in breast cancer mortality which did not quite achieve statistical significance (p = 0.07) while those with a hysterectomy who received CEE alone had 45% statistically significant reduction in breast cancer (p=0.02). Thus, the breast cancer mortality through 18 years absolutely mimicked the breast cancer incidence through 11.7 years.
In choosing a SERM to pair with CEE for endometrial protection, one looks at studies in experimental animals that have been oophorectomized, given various SERMs, then sacrificed, and uterine wet weight measured. Some SERMS are more agonistic (tamoxifen, levormeloxifene) while others are more antagonistic [18]. As mentioned above, bazedoxifene is the only SERM that has ever been shown to reduce endometrial thickness in postmenopausal women. When bazedoxifene is combined with CEE, experimental animal data looking at mammary duct elongation and end bud proliferation showed it to act like the control oophorectomized animal without any proliferation or elongation. The combination of estradiol with bazedoxifene resulted in some end bud proliferation whereas estradiol by itself resulted in marked end bud proliferation and mammary duct elongation [19].
Subsequently, this combination of CEE and bazedoxifene was tested in approximately 7,500 women through five Phase 3 trials. The mean daily frequency of hot flashes was reduced 80% compared to placebo (p < 0.001) and the average daily hot flash severity score was reduced 52% (p < 0.001). This reduction was seen as early as four weeks and carried through the full twelve weeks of the efficacy trials. Furthermore, over a 24-month period of time, both in women within 1 to 5 years of their last menstrual period and women greater than five years since their last menstrual period, there was a statistically significant improvement in maintenance of bone mineral density both at the lumbar spine and total hip compared to placebo (p < 0.01) at 6, 12, 18, and 24 months [20].
In 2013, this TSEC combination of CEE/ bazedoxifene was approved for the treatment of vasomotor symptoms and the prevention of osteoporosis.
Previously, there had been an attempt to combine estradiol 1 mg/day + raloxifene 60 mg/day in 123 postmenopausal women in a planned study for 52 weeks [21]. That study was stopped early as the authors concluded, “because signs of endometrial stimulation were seen in the combination group, we do not recommend concurrent clinical use of oral 17-beta estradiol 1 mg/day and raloxifene.”
Ospemifene
Until the development of ospemifene, an oral SERM, all available vulvovaginal atrophy (VVA)/genitourinary syndrome of menopause (GSM) treatments were systemic or local steroid hormones such as estradiol, conjugated estrogens, and dehydroepiandrosterone (DHEA). Fear of estrogens because of, “class labeling” as well as the inconvenience of vaginal administration had limited utilization for some women.
Ospemifene is a third generation SERM that was originally being developed for osteoporosis and was found to have estrogenic effects on vaginal tissue (as well as bone and lipids like all other SERMS) while remaining antiestrogenic or neutral in the breast and endometrium, respectively [21]. Multiple Phase 3 placebo-controlled trials [22,23] showed that, compared to placebo, ospemifene improved superficial cells, reduced parabasal cells on maturation index, lowered vaginal pH, and improved most bothersome symptoms, originally dyspareunia and then later vaginal dryness. The ACOG has endorsed ospemifene with level A evidence as a first line therapy for dyspareunia noting absent endometrial stimulation [24]. While its approval is for VVA/GSM, ospemifene will have positive effects in bone and breast. Clinical data from three Phase 1 or Phase 2 clinical trials showed that ospemifene 60 mg/day had a positive effect on bone turnover biochemical markers in healthy postmenopausal women with significant improvements relative to placebo and effects comparable to those of raloxifene [25].
In terms of breast, ospemifene inhibits breast cancer cell growth in in vitro cultures as well as experimental animals [26]. In a claims database study comparing ospemifene users and untreated controls, no difference was found in breast cancer incidence nor recurrence rates in ospemifene users compared with matched controls [27].
Thus, although one would not use ospemifene primarily for breast cancer chemo prevention, or prevention or treatment of osteoporosis, in choosing an agent for VVA/GSM, attention to any potential add-on benefit to bone and breast for that individual patient would be totally appropriate. Another way to express this is that the direction of activity of ospemifene in bone and breast is indisputable as it is a SERM class effect. The magnitude of this activity has not been studied.
Summary
SERMs are unique compounds that can have varied activity in various tissues. Unlike estrogens, they do not display class effects and the labeling for each SERM is somewhat different as is the indication. Understanding the differences between various SERMs and their potential benefits are essential for healthcare providers to assist their patients in optimizing healthy aging through the menopause transition and beyond.
Declaration of interest: The author declares having no conflicts of interest.