Introduction
Background
Premature ovarian insufficiency (POI) is a clinical syndrome characterized by the loss of ovarian activity before the age of 40; it is associated with hypoestrogenism and oligo- or amenorrhea 1. POI can have different etiopathogenetic causes: it can be iatrogenic, as after chemotherapy and radiotherapy or surgery, but it can also be associated with chromosomal/genetic defects (Turner syndrome or fragile X syndrome) and with autoimmune disorders, or it can be idiopathic 2. Hypergonadotropic amenorrhea also occurs in women with complete androgen insensitivity syndrome (CAIS) who have undergone gonadectomy. CAIS is the most common 46,XY disorder of sex development, and it is caused by mutations in the androgen receptor causing a complete resistance to the action of androgens 3.
Both POI and CAIS after gonadectomy are conditions associated with a high risk of bone health problems. POI is known to be associated with lower bone density than is observed in healthy controls 1,4–6 and with increased fracture risk in later life 7. Adolescents and adults with CAIS and removed gonads present reduced bone mineral density (BMD) mainly in the lumbar region; 8–13 however, adequate hormonal therapy (HT) seems to be able to improve BMD at lumbar level at least 6,9,11.
In women with POI and in gonadectomized CAIS patients, HT is strongly recommended as it plays a significant role in bone protection and also in the prevention of cardiovascular disease and mortality 1. There is still little evidence on the optimal dose, regimen and type of HT in young women with absent ovarian estrogen production, 6,14–17 and in particular data are still lacking on oral administration of estradiol valerate.
Objectives
The primary aim of this retrospective pilot study was to assess the effects of different estrogenic molecules and routes of administration (oral estradiol valerate, transdermal estradiol, oral ethinylestradiol) on the bone health of young women with hypergonadotropic amenorrhea (idiopathic or iatrogenic POI, women with POI and Turner syndrome or CAIS) and to compare them to the outcomes of women receiving no treatment. The secondary aim of our study was to evaluate the effects of these treatments on biochemical and clinical characteristics of these patients.
Material and methods
Study design and population
In this retrospective pilot study, we evaluated young adults with secondary hypergonadotropic amenorrhea. Women were selected from patients who attended the Gynecology and the Physiopathology of Human Reproduction Unit at S. Orsola University Hospital in Bologna. In this study we included women with a normal 46,XX karyotype with POI, gonadectomized women with CAIS, and women with Turner syndrome. All the CAIS patients had a 46,XY karyotype with confirmed androgen receptor mutation and previous bilateral gonadectomy, while the Turner syndrome patients presented a 45,X0 karyotype.
Inclusion criteria were: at least six months of amenorrhea with confirmed hypoestrogenism; a follicle-stimulating hormone (FSH) level of over 30 IU/L recorded on at least two occasions, four weeks apart; and at least two DXA evaluations performed 12–36 months apart.
Exclusion criteria were: amenorrhea without confirmed hypoestrogenism, change of estrogenic treatment during the study period, other concomitant treatments for osteoporosis or treatments capable of affecting bone density, bodyweight change of over 10% during the study period, and diagnosis of an eating disorder. We also excluded CAIS subjects with previous testosterone intake and intact gonads.
All participants were in good physical health at the time of enrollment and were asked about previous illnesses and medication use, current and past smoking habits.
The following data were recorded for all patient groups: age at amenorrhea onset, age at starting estrogens, type and dose of estrogens, and route of administration (oral vs. transdermal). For CAIS subjects, age at gonadectomy was also recorded.
The patients were divided into four study groups according to treatment: transdermal estradiol in gel or patch (ttsE2), oral estradiol valerate (oE2), oral ethinylestradiol (EE) with or without a progestin, or no treatment (noT).
We compared spine and femoral BMD in these four groups. All patients underwent clinical, laboratory and radiologic assessment as per clinical practice. Anthropometric measurements were taken from all subjects: stature was measured with a stadiometer as the distance from the vertex to the floor by asking the subject to stand erect, barefoot with their shoulders touching the wall. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
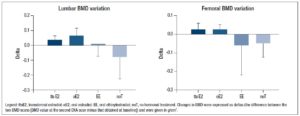
Bone parameters were noted in the patients’ charts. BMD was measured by dual X-ray absorptiometry. Spinal BMD was obtained between lumbar levels 1–4 (L1–L4) and total hip BMD at the femoral neck, trochanteric and intertrochanteric regions. The results of BMD measurement were recorded in g/cm2. With regard to BMD, the T-score indicates the extent to which BMD measured in a bone site differs from that of the reference sample, consisting of healthy subjects aged 25–30 years (the age at which peak bone mass is reached). The World Health Organization (WHO) criteria divide BMD T-scores as follows: normal: between +2.5 and 1.0 SD compared to the average peak bone mass value in a young adult; osteopenia: between –1 and –2.5 SD, and osteoporosis: below –2.5 SD. BMD Z-scores, on the other hand, refer to the number of standard deviations of values measured in bone sites and how they deviate from those measured in the healthy reference population, consisting of subjects of the same sex and age as the patients being studied. Changes in BMD were also expressed as deltas (the difference between the two BMD scans [BMD value at the second DXA scan minus that obtained at baseline]).
The following laboratory test results were recorded: estradiol, luteinizing hormone, FSH, fasting glucose, total cholesterol (Tot Chol), high- and low-density lipoproteins (HDL and LDL), triglycerides (TG), aspartate and alanine aminotransferases, prothrombin time, and activated partial thromboplastin time.
This study was approved by the Medical Ethics Committee of S. Orsola Hospital, Bologna, and all the participating subjects gave their written informed consent. All patients were over 18 years of age at the time of their inclusion in the study.
Statistical analysis
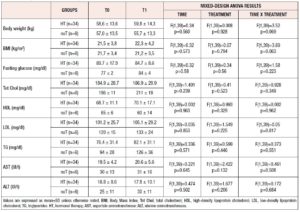
All continuous data are expressed as mean and standard deviation of the mean, and all categorical data are expressed by frequency rate and percentage. The Kolgomorov-Smirnov test was used to assess the normality of distributions. When data were normally distributed, the following was used to assess differences between more than two groups: one-way ANOVA with post-hoc pairwise comparisons with Tukey’s test. Otherwise, the Kruskal-Wallis test was used. Pearson's nonparametric chi-squared test or Fisher's nonparametric chi-squared test was performed to investigate the relationships between categorical variables. A mixed-design ANOVA with post-hoc pairwise comparisons with Tukey’s honest significance difference test was conducted to compare the main effects and the effect of the interaction between type of treatment and time on laboratory and DXA parameters. For all tests, p<0.05 was considered significant. Considering BMD variations in a previous study, a sample size of 58 subjects was calculated to achieve 80% power and 5% probability of type I error, however, the enrolment rate in this pilot trial was affected by the rarity of the described condition. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) software, version 23.0 (International Business Machines Corp. Armonk, NY).
Results
Clinical characteristics of enrolled women
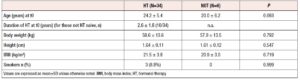
Sixty-two women with amenorrhea were potentially eligible and were screened. In accordance with our inclusion-exclusion criteria, 22 women were excluded because of missing DXA data. Therefore 40 women were enrolled and included in the data analysis. Their mean age at the time of the first available DXA evaluation (t0) was 23.8 ± 5.5 years (range: 15–35 years). Etiopathogenetic factors for amenorrhea were distributed as follows: 22 women with CAIS, 10 women with Turner syndrome, and eight with idiopathic (five patients) or iatrogenic POI (three patients, after chemotherapy and/or radiation for cancer treatment), the latter with 46,XX karyotype. The mean duration of amenorrhea before the start of HT was 13.2±4.1 months.
In our cohort, six women received no treatment (noT, n=6) while 34 women were treated with the different regimens: ttsE2 (n=12), oE2 (n=15), EE (n=7). In the ttE2 group, six women used transdermal estradiol gel 2 mg and six used transdermal patch containing 50 mcg estradiol and 10 mcg levonorgestrel; in the oE2 group, all patients used 2 mg estradiol valerate [nine women used only estradiol valerate while six, having a uterus, used estradiol valerate 2 mg plus dienogest (KlairaÒ)]. In the EE users, the dosage of EE ranged from 20 to 30 mcg and the associated progestin in the majority of cases was drospirenone; the administration was cyclic with 21/7 or 24/4 regimens. The patients in the noT group came to our center at the time of the second DXA evaluation and were not using HT because of non-compliance with a previous prescription or no previous prescription.
Clinical characteristics of the patients at the time of their first DXA scan,are presented in tables 1 and 2. All participants in the study were Caucasian and born in Italy.
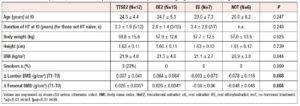
At t0 (first DXA scan) 30 patients (75%) were hormone naïve, while 10 patients (25%) underwent their first DXA scan after the start of treatment (mean duration of HT at baseline is reported in Tables 1 and 2).
Smoking rates were not different between groups. Hepatic enzymes were in normal ranges for all included subjects (data not shown). The groups were homogeneous with no significant differences in the evaluated anthropometric and metabolic parameters, except for LDL-cholesterol, which showed significantly higher levels in the EE vs. the oE2 group (133 ±41 mg vs. 88 ± 22 mg, p=0.01).
Bone parameters
Bone parameters at the time of the baseline DXA scan (t0)
In the whole cohort, lumbar BMD at first DXA evaluation was 0.913 ± 0.131, with a T-score of −1.92 ± 1.04 and a Z-score of −1.81 ± 0.98. Femoral BMD was 0.865 ± 0.125, with a T-score of −1.06 ± 0.99 and a Z-score of −1.02 ± 0.90. Sill considering the whole cohort, 30/40 patients (75%) presented osteopenia and 8/40 (20%) osteoporosis in at least one bone site. No osteoporotic fractures were detected in the study groups. At t0, lumbar and femoral BMD were not significantly different between the four groups. Also, the majority of the 10 women already using HT at the time of their first DXA scan had impairment of BMD, with three subjects presenting osteoporosis (two in the ttsE2 and one in the oE2 group) and six osteopenia (three in the ttsE2, one in the oE2, and one in the EE group) in at least one bone site. Two had normal BMD in all bone sites.
Bone parameter variations in women undergoing different hormonal regimens
The time interval between the two DXA evaluations (t1–t0) was 22.1 ± 9.2 months, with no significant differences between the four groups (DXA timing interval was 20.6 ± 8.9 months for ttsE2, 22.9 ± 9.3 months for oE2, 20.3 ± 8.8 months for EE, and 25.0 ± 9.5 months for noT). All the patients using HT reported correct and continuous use of their treatment between the two DXA evaluations without any interruptions or changes in molecules or dosing.
Lumbar BMD showed a significant time-treatment interaction effect (p=0.004] (Table 3). Changes in BMD from t0 to t1 (delta) were significantly different between treatment groups (p=0.008) (Table 2 and Figure 1). The post-hoc analysis revealed a significant increase in lumbar BMD in the oE2 group when compared with noT (p= 0.006) and in the ttsE2 group versus noT (p=0.036).
Also, femoral BMD showed a significant time-treatment interaction effect (p=0.025] (Table 3). Changes in BMD from t0 and t1 (deltas) were significantly different between groups (p=0.004), with significant variation (increase) in femoral BMD in both the ttsE2 and the oE2 group when compared with noT or with EE (Table 2).
Anthropometric and metabolic parameters
BMI at baseline was within the normal range and similar in all groups. BMI and metabolic parameters (glucose, Tot Chol, HDL and LDL cholesterol, TG and hepatic function) did not show significant variations in treated or not treated patients, in whom no significant treatment, time or interaction effects were found. Arterial pressure was within normal ranges and did not change during the study period.
Discussion
In this pilot study, we compared changes in bone, metabolic and anthropometric parameters in very young women with hypergonadotropic hypogonadism, POI and CAIS undergoing different hormonal regimens (oral estradiol valerate, transdermal estradiol, oral ethinylestradiol or no hormonal treatment). Even though our results should be confirmed in a larger sample, both transdermal and oral administrations of estradiol seemed to be associated with a greater short-term improvement in bone BMD when compared to ethinylestradiol or no treatment.
At the time of their first DXA scan, the whole cohort presented T-score values suggesting osteopenia, in particular for the lumbar but also for the femoral site, with only five percent of patients presenting normal BMD in all bone sites. The second DXA scan was performed 22.1 ± 9.2 months after the first. Both oral estradiol valerate and transdermal estradiol were associated with a significant increase in lumbar BMD and with changes in femoral BMD, significantly different from those obtained with oral ethinylestradiol or no treatment. Women treated with oral ethinylestradiol or receiving no treatment showed subtle reductions or no significant changes in BMD. No significant changes in anthropometric and metabolic parameters were noted throughout the treatment period, even though the small sample size might have limited the detection of differences.
POI and CAIS are known risk factors for bone health. Several studies have demonstrated that women with POI have lower BMD and a significantly increased fracture risk 7,18,19. Long-lasting HT is able to reduce this increased fracture risk 4,17,20–22. In particular, a significant health concern for very young women with POI is that the potentially prolonged estrogen deficiency at a young age can lead to a decreased peak bone mass accrual in the case of delayed or inadequate HT 4. Similarly, women living with CAIS are known to have low BMD both before and after gonadectomy because of a combination of estrogen deficiency and bone resistance to androgens, and in some cases because of inadequate HT after gonadectomy 9,11,12,23. The lumbar spine is the site more prone to BMD deficit as it is characterized by a prevalence of trabecular bone. We recently confirmed that HT containing estrogens is able to increase BMD at this site but not at the femoral neck 6.
Research on the optimal hormonal treatment for women with POI and CAIS is still limited. In our cohort, the transdermal route was confirmed to be superior to oral administration of ethinylestradiol in the improvement of lumbar and femoral BMD. Transdermal administration of estrogens has already been associated with better BMD values in women with POI when compared both with conjugated oral estrogens and with oral ethinylestradiol 15,21,24. In the CAIS population, the transdermal route of estradiol administration seems to be superior to oral administration in terms of the increase in total body BMD 6. This difference can be explained by the effect of the treatment on the secretion of insulin-like growth factor 1, which is a bone-trophic hormone downregulated only by oral administration but not by transdermal administration 25.
Albeit with the limit of the small sample size, our study seems to show that oral estradiol valerate is superior to oral ethinylestradiol in increasing lumbar and femoral BMD. This is the first study to detect the ability of oral estradiol valerate 2 mg, combined or not with a progestin, to increase BMD more than oral ethinylestradiol plus a progestin: the BMD increase at the lumbar spine was similar to that obtained by transdermal estradiol administration and even greater than that obtained via the transdermal route at the femoral neck.
One of the reasons for our finding may be the different estrogenic molecules considered (estradiol valerate vs ethinylestradiol), and even though data on this topic are scarce they may have different tissue effects and/or different impact on liver metabolism26. Estradiol valerate in association with dienogest was reported to have no short-term (six months) adverse effect on bone turnover markers, maintaining stable BMD values in young, healthy, fertile women 27, but its effects in women with POI have never been assessed. Limited but consistent data are available regarding estradiol in this population: Cartwright et al., in a two-year open randomized trial, reported the superiority of estradiol 2mg (plus levonorgestrel 5mcg for 12 days a month) over ethinylestradiol 30 mcg (plus levonorgestrel 150mcg taken daily for 21 days a month) in increasing lumbar spine BMD in women with spontaneous POI 17.
Different progestins may also have played a role in BMD variation in our cohort. Our cohort of patients using combined estro-progestinic therapy was too small to detect specific effects associated with different progestins. However, we should keep in mind that progesterone is able to increase mature osteoblasts and the production of collagen bone matrix 28,29; furthermore progesterone plus estrogens has been demonstrated to be superior to estrogens alone in increasing lumbar BMD 30,31.
Some limitations of this study should be acknowledged. First of all, POI at this young age and CAIS are rare conditions; for this reason, this pilot study analyzed a small cohort, and this may have led to a statistical type II error. Furthermore, different etiologies of amenorrhea were included in the analysis. Also, some statistically significant associations should be interpreted with caution considering the small number of subjects in each group. The retrospective nature of the study is a further limitation. The two DXA evaluations were performed with the same machine for each patient, however in the cohort as a whole different machines were used.
In conclusion, these preliminary data suggest the superiority of oral estradiol valerate over oral ethinylestradiol in improving lumbar and femoral BMD in young women with hypergonadotropic hypogonadism. Consistent with previous literature, our cohort of women with POI and CAIS was characterized by some impairment of BMD in particular at the lumbar level. After 22.1 ± 9.2 months, treatment with oral estradiol valerate or transdermal estradiol was shown to be able to induce positive changes at lumbar and femoral sites, whereas women treated with oral ethinylestradiol or not receiving any treatment showed subtle reductions or no significant changes in BMD. Further studies in larger cohorts are mandatory to understand which hormonal treatment is the best for bone health in young women with POI or CAIS as there is little evidence on the optimal molecules, regimen and doses.
Acknowledgements
The authors thank Julie Norbury for English copy editing.
Disclosure Statement:
The authors declare that there is no conflict of interest.