Introduction
Adrenal gland secreted stress hormones such as cortisol & DHEA-S play an important role during pregnancy and parturition [1-4]. However, imbalance of maternal cortisol levels due to prenatal stress or adrenal insufficiency can lead to complications in pregnancy and poor birth outcome, including preterm birth and infants born with low birth weight [3]. Some studies have demonstrated that external administration of DHEA lead to lowered miscarriage rates in women with reduced ovary functions [5,6]. Therefore, it is of vital importance to monitor the concentration of these hormones during pregnancy for assessing the well-being of both mother and the growing fetus.
Reference range-based assessment of hormones has been utilized for screening endocrine associated disorders [7,8]. With respect to non-pregnant women, reference range of cortisol and DHEA-S in plasma or saliva has been well established [9-13]. Unlike DHEA-S, cortisol exhibits strong diurnal variation, with concentrations rising rapidly after awakening and subsequently decreasing through the day [14, 15]. Several independent studies have evaluated concentrations of cortisol and DHEA-S during pregnancy [16-19]. However, unlike non-pregnant women-validated, consistent, and clear-cut reference range for these hormones in plasma and saliva samples from pregnant women is lacking. This may be due to the differences in sample collection method, choice of methods, immunoassay manufacturer, geographical location, and other factors [16].
Quartile scoring of cortisol and DHEA-S concentrations among pregnant women can avoid reference range associated limitations. This method involves dividing the hormone concentrations in the given population into quartiles and assessing the association of individual quartiles with various parameters [20-23]. This methodology has been applied for understanding the association of DHEA-S with parameters such as bone mineral density, cardiovascular mortality among older men and mortality among older women [24-26]. Likewise, quartile score- based categorization of maternal cortisol concentrations has also been performed to better understand the association of cortisol with infant low birth weight, new-born stunting and right amygdala volumes at 7 years of age [27, 28].
Although studies have assessed the association of individual prenatal stress hormones with birth outcome, effects of combinatorial facets of these hormones on birth outcome have not yet been evaluated. In addition, association of combinatorial characteristics of cortisol or DHEA-S in varied biological sources such as plasma and saliva with birth outcome is not clear. Biological rationale for performing such combinatorial analyses of prenatal stress hormones stems from the following known features: 1) cortisol and DHEA-S regulate each other during pregnancy through adrenocorticotropic hormone and cortisone or DHEA mediated mechanisms [1, 29], 2) cortisol and DHEA-S enter saliva through differential mechanisms [30]. Unlike non-pregnant women, salivary and circulatory cortisol levels do not correlate with each other [31, Sundararajan A et al., unpublished] and 3) compared to non-pregnant women, the metabolic clearance rate of circulatory DHEA-S in normal pregnant women is much higher [32] as circulatory DHEA-S is diverted to placenta for estrogen production [1]. As a result, circulatory DHEA-S levels decrease drastically as pregnancy progresses [17,18]. Parallel salivary hormones associated metabolic clearance or entry rate pattern during normal and poor birth outcome associated pregnancy is unclear. Therefore, there is a need to develop novel approaches for data analysis to understand association of changes in concentrations of these hormones during pregnancy and its combined effects on birth outcome. Developing such methods of data analysis will provide the foundation for designing a comprehensive panel of prenatal stress hormones-based models for efficiently predicting poor birth outcome among pregnant women.
Overall, the aim of this study was to develop a methodology of data analysis to assess the individual and combinatorial associations of circulatory and salivary prenatal stress hormones with birth outcome. This was achieved by creating combinatorial categories of cortisol & DHEA-S in plasma and saliva of pregnant women through quartile scoring approach and straightforward Microsoft Excel formulae. Individual and combinatorial analysis were compared. An effective and practical approach to generate combinations of stress hormones in plasma and saliva unique to each pregnant woman has been developed that could predict poor birth outcome among pregnant women.
Methods
Study area and subject recruitment
This study was conducted in the tribal regions of Bhiloda and Modasa blocks of Aravalli district of Gujarat, India for a period of two years (March 2018-March 2020). During this period, pregnant women in their first or second trimester were enrolled with their informed consent. Subject recruitment was done through collaboration with local government and private sector health care providers. Details of study area, pregnant women enrolment and characteristics can be found in published study methodology [33]. This study has been approved by institutional ethics committee.
Sample collection, processing, and storage
Blood and saliva samples were collected by trained health care workers from enrolled pregnant women at government and private hospitals in the tribal region. Blood samples were collected by venepuncture and saliva samples were collected by passive drooling. Samples were stored and transported to the institutional research laboratory under cold conditions (4°C). To circumvent the issue of diurnal variation exhibited by cortisol, all samples in the study were collected within a close time zone (late morning-early afternoon). In addition, both blood and saliva samples from each participant were collected at the same time. Owing to logistical restrictions, samples from the same pregnant woman at multiple time points within the day could not be collected. Sample quality was assessed before further processing. Any visible blood contaminated saliva samples and lysed blood samples were not included in the study. Blood samples were centrifuged at 2.000rpm for 15min and plasma was collected, stored at -800C until further use. Saliva samples were centrifuged at 4.000rpm for 5min to get rid of mucus and food debris. The supernatant was collected and stored at -800C until further use.
Quantitative estimation of cortisol & DHEA-S by ELISA assay
Commercially available ELISA assays (DiaMetra, Italy) based on competitive immunoenzymatic colorimetric methodology was used for the quantitative estimation of cortisol and DHEA-S concentrations in plasma and saliva samples. Repeated freeze-thawing was avoided. All ELISA assays, including the downstream estimation of individual hormone concentrations with the help of standard curves were performed as per manufacturer’s instructions. Concentrations of cortisol in plasma and saliva, DHEA-S in saliva were expressed as ng/ml. DHEA-S concentrations in plasma was expressed as µg/ml.
Quartile score-based categorization
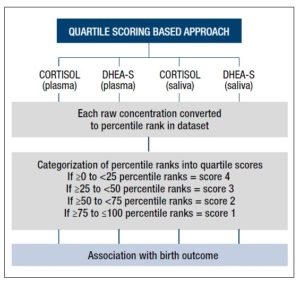
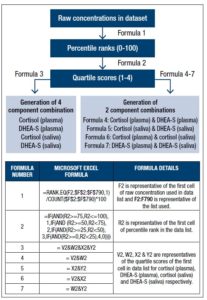
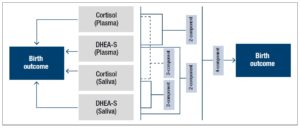
Individual cortisol and DHEA-S concentrations derived from each pregnant woman in the dataset were assigned a percentile rank within the respective group of plasma or saliva samples. These percentile ranks were further categorized into quartile scores signifying a specific range of percentile rank (≥0 to <25 = score 4, ≥25 to <50 = score 3, ≥50 to <75 = score 2, ≥75 to ≤100 = score 1). Individual quartile scores were directly assessed for their association with birth outcome (Fig 1). Microsoft excel formulae were used to generate quartile scores (Supplementary Fig 1).
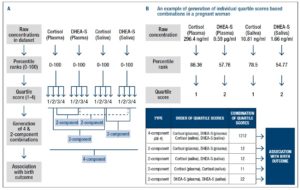
Furthermore, each pregnant woman-specific quartile score combinations were generated to assess association of salivary and circulatory cortisol & DHEA-S with birth outcome (Fig 2). Microsoft excel formulae for each step are described in the supplementary figure 1. First set of unique combination included all the 4 components in the order of cortisol & DHEA-S in plasma & saliva. The 4-component category was created to provide an overview of the status of stress hormones in each pregnant woman and to assess their combined association with poor birth outcome. Other sets of unique combinations included the 2-components in the order of a) Cortisol & DHEA-S in plasma, b) Cortisol & DHEA-S in saliva, c) Cortisol in plasma and saliva and d) DHEA-S in plasma and saliva (Fig 2A).
For example, a pregnant woman with quartile scores of 1 for cortisol in plasma and saliva; scores of 2 for DHEA-S in plasma and saliva was given the 4-component combination score of 1212, 2-component combination scores of 12 for both cortisol-DHEA-S in plasma & saliva, 11 for cortisol in plasma and saliva, 22 for DHEA-S in plasma and saliva (Fig 2B).
Pregnant women categorization based on birth outcome
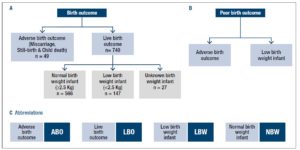
In the study, pregnant women were categorized based on birth outcome into adverse birth outcome (n=49) or live birth outcome (n=740). Adverse birth outcome cases included pregnant women who delivered infant with conditions of still birth, abortion, death, and other abnormalities (Fig 3A). Pregnant women with live birth outcome were further categorized into normal birth weight infants (n=566), low birth weight infants (n=147) and unknown birth weight infants (n=27), based on birth weight data of infants determined post-delivery. Infant was considered normal birth weight if ≥ 2.5 Kg and considered low birth weight if <2.5 Kg; criteria as defined by WHO [34]. Overall, the term poor birth outcome in the study included adverse birth outcome and low birth weight of infant associated cases (Fig. 3B). Following abbreviations were used to describe birth outcome in the study (Fig 3C):
- Adverse birth outcome (ABO),
- Live birth outcome (LBO),
- Low birth weight infant outcome (LBW),
- Normal birth weight infant outcome (NBW).
Pregnant women with unknown birth weight infants were excluded from LBW associated analysis. However, they were included for ABO or LBO associated analysis. Pregnant women having both ABO and LBW birth outcome were grouped under ABO category only and excluded from the LBW category.
Statistical analyses
Microsoft Excel was used to generate individual and combinations of stress hormones in plasma and saliva datasets based on quartile scoring approach. IBM SPSS Statistics 20 was used to determine percentage of women exhibiting various combinations and to determine bivariate logistic regression for Odds Ratio (OR) calculation. OR estimation through logistic regression analysis provided the statistical methodology to significantly assess the occurrence of ABO or LBW event in higher individual components or the unique combinations of prenatal stress hormones in plasma-saliva. Relevant lowest individual component or combination(s) were used as reference category.
Results
Profile of prenatal stress hormones generated through novel quartile scoring approach
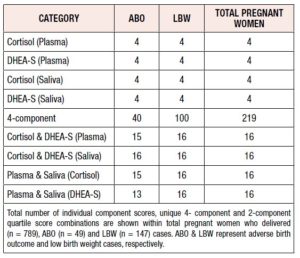
A bird’s eye view of total unique combinations of prenatal stress hormones was compared within the groups of ABO, LBW cases and total pregnant women with known birth outcome. Single component analysis generated 4 categories with the four individual quartile scores. In contrast, in the 4-component category, 219 unique combinations were observed in the total pregnant women following quartile score-based combinatorial analyses. Only 40 and 100 combinations were observed among ABO and LBW cases, respectively. 16 unique combinations were observed in total pregnant women with respect to 2-component categories. Unlike ABO cases, all 16 combinations were observed among LBW cases in each of the 2- component category (Table 1).
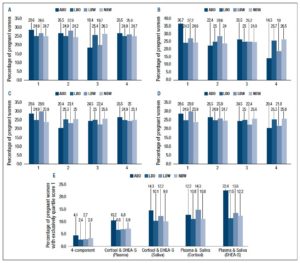
Quartile score-based analysis of individual prenatal stress hormones in plasma or saliva revealed increased representation of pregnant women with ABO exhibiting quartile score 1 (75th-100th percentile) for circulatory DHEA-S, compared to women with LBO (Fig 4B). Compared to NBW, LBW cases consistently demonstrated increased representation of higher quartile score(s) 1 or/and 2 for prenatal stress hormones (Fig 4A-D).
The novel combinatorial methodology generated more insight into the profile of prenatal stress hormones, compared to individual prenatal stress hormone analyses. Overall, ABO cases consistently demonstrated increased percentage of women exhibiting quartile score 1 combinations in the 4-and 2-component categories, compared to corresponding LBO cases (Fig 4E). A prominent increase in the percentage of women exhibiting highest quartile score combination 11 for both circulatory and salivary DHEA-S was observed in ABO cases, compared to women with LBO. In comparison to NBW, an increased representation of quartile score 1 combinations was observed among LBW cases in all categories, except for the 4-component and circulatory cortisol-DHEA-S categories (Fig 4E). Exploring percentage of pregnant women with exclusively quartile score 1 combinations has been shown as an example of this methodology. However, there are other quartile score combinations that could be of significance to ABO or LBW outcome as can be seen in the following section on Odds Ratio estimations.
Interestingly, majority of pregnant women with ABO and LBW outcome demonstrated highest quartile score(s) of prenatal stress hormones in both individual and combinatorial analysis. In contrast, majority of pregnant women with LBO and NBW outcome demonstrated lowest or lower quartile score(s) of prenatal stress hormones (data not shown). Detailed assessment and composition of all the generated combinations of prenatal stress hormones are beyond the scope of the methodology emphasis and will be explored in subsequent manuscript.
Association of ABO and LBW with prenatal stress hormone profiles generated through quartile score-based categorization
Odds Ratio (OR) was determined through binary logistic regression analyses for establishing the odds of ABO or LBW occurring in pregnant women with higher quartile scores of stress hormones compared to pregnant women with the relevant lowest quartile score(s) (Tables 2, 3). Only those combinations that yielded significant results (p<0.05) for OR have been shown.
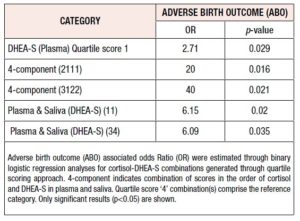
Among individual component categories, women with the quartile score ‘1’ for circulatory DHEA-S demonstrated higher odds of ABO event occurrence, compared to women with quartile score ‘4’ in the same category (Table 2). The combinatorial analysis demonstrated that pregnant women with either of the two 4- component combinations (2111, 3122) exhibited significantly and remarkably high odds of ABO event occurrence, compared to women with 4444 score in the same category. Pregnant women with either of the two 2-component combinations in the indicated categories also demonstrated higher odds of ABO event occurrence compared to women with 44 score in the same category (Table 2).
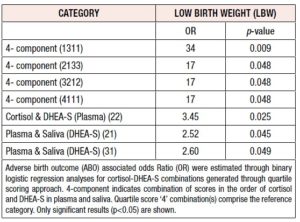
In contrast to ABO cases, none of the individual higher quartile scores for prenatal stress hormones in plasma or saliva demonstrated significant association with LBW outcome. However, the combinatorial analysis revealed significantly higher odds for LBW outcome in pregnant women with four of the 4-component and three of the 2-component categories, compared to pregnant women with 4444 and 44 score combinations respectively (Table 3).
Discussion
Cortisol and DHEA-S play important roles in maintaining successful pregnancy and imbalance in these hormones can lead to complications such as preterm birth and low birth weight. Therefore, regular quantitative analysis of these hormones during pregnancy is of vital importance. Although individual roles of stress hormones during pregnancy are well understood, combined effects of these hormones are not clear. Along these lines, the inter-relation of salivary and circulatory stress hormones in predicting poor birth outcome is not very well defined. In this direction, our study has developed a methodology for building a combinatorial and individual profile of pre-natal circulatory- salivary cortisol and DHEA-S unique to each pregnant woman in the population (Fig 5). This methodology of combinatorial assessment of stress hormones for each pregnant woman provides a unique insight into the interplay of circulatory and salivary cortisol-DHEA-S during pregnancy and poor birth outcome. Such a comprehensive profile of prenatal stress hormones could be a launching pad for predicting the occurrence of adverse birth outcome or low birth weight among pregnant women.
This methodology could be used as a non-biased approach to draw preliminary biological interpretations of the interplay of stress hormones during pregnancy and birth outcome. However, larger prospective studies within Indian population need to be explored further to confirm the significance of this study identified unique combinations.
Advantages of combinatorial assessment, in comparison to individual analysis was observed. For example, among ABO cases, when individual hormones were assessed, quartile score 1 of circulatory DHEA-S was represented more compared to LBO cases. However, combinatorial analysis revealed that among these ABO cases, there was a higher percentage of women with quartile score of 1 for circulatory DHEA-S, in extension with quartile score of 1 for salivary DHEA-S. Therefore, combinatorial analysis provided more insight into the profile of prenatal stress hormones.
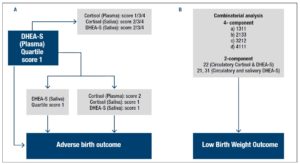
The novel combinatorial methodology also offered a means to confirm and strengthen the results of the association of stress hormones with birth outcome. For example, women with unique quartile score combination of 2111 in the 4-component category demonstrated significant and remarkably high odds (20) of ABO event occurrence, compared to women with the quartile score combination of 4444 in the same category. A portion of this result was reiterated in the a) 2- component category of circulatory and DHEA-S, in which women with quartile score of 11 demonstrated significant odds of ABO event occurrence and b) individual hormone category of circulatory DHEA-S, in which women with quartile score of 1 demonstrated significant odds of ABO event occurrence.
Along these lines, combinatorial analysis also offered selective advantage over individual analysis (Fig 6). For example, individual hormone analysis demonstrated that women with quartile score 1 for DHEA-S in plasma have higher odds of ABO event occurrence. However, from a practical perspective, among pregnant women in the population, quartile score 1 could co-exist in combination with quartile scores 4, 3, 2 or 1 for salivary DHEA-S. The combinatorial analysis revealed that only the combination of quartile score 1 for DHEA-S in plasma with score 1 for salivary DHEA-S, and furthermore specifically with score 2 for circulatory cortisol and score 1 for salivary cortisol demonstrated higher odds for ABO event occurrence (Fig 6A). Likewise, none of the single component analysis demonstrated significant association with LBW outcome. However, combinatorial analysis revealed 4- and 2- component combinations that are significantly associated with LBW outcome (Fig 6B).
Although the Microsoft Excel formulae used for combinatorial analysis is straightforward and easy to implement, cost of the combinatorial analysis through ELISA assay increases as multiple components are added to the analysis. However, this limitation could be evaded by developing multiplex ELISA assays that detect both cortisol and DHEA-S simultaneously. Cortisol and DHEA-S could be assessed in other biological sources such as hair and fingernails to estimate chronic stress [35]. Therefore, the combinatorial analysis described in this study using plasma and saliva could be extended to other biological sources to obtain a comprehensive picture of prenatal stress.
Strengths of this study includes the reasonably large and randomized prospective design, with measures of stress hormones in plasma and saliva from each participant and high rates of follow-up on birth outcome. In addition, all the participants are from the tribal region, with largely similar socio-economic conditions, thereby minimizing the potential for confounding the effects on stress hormones. Limitations in the design of the study includes resource and logistical constraints in the field for sample collection from pregnant women multiple times through the day to account for diurnal variation of cortisol. An attempt was made to control for this variation by collecting both plasma and saliva together, around the same time zone of late morning to early afternoon. In contrast to cortisol, DHEA-S does not demonstrate diurnal variation and meaningful biological interpretations could be made for predicting the risk of poor birth outcome among pregnant women.
In conclusion, the study has developed a novel quartile scoring approach for analysing salivary and circulatory prenatal cortisol and DHEA-S, given the lack of validated reference range for these hormones during pregnancy. Study has importantly identified unique combinations of quartile scores depicting range of concentrations of cortisol and DHEA-S that are significantly associated with adverse birth outcome or low birth weight outcome among pregnant women. In addition, none of these significant quartile score combinations were common among the two types of poor birth outcome, indicating potentially differential roles for cortisol and DHEA-S in regulating adverse birth or low birth weight outcome. Overall, study concludes that a combinatorial, rather than a single component analysis with respect to prenatal salivary and circulatory cortisol-DHEA-S offers a deeper and more sensitive insight into predicting birth outcome.
Supplementary Figure 1 shows details of Microsoft Excel formulae used to generate individual and combinations of prenatal stress hormones in plasma- saliva of pregnant women through quartile scoring approach.
Acknowledgements
We acknowledge the efforts of data collectors and research laboratory personnel for assisting with the sample collection from study sites and transportation to the research laboratory.
Funding
The research was funded by SEED of Department of Science and Technology, Govt. of India grant. Funder has no inputs in design, implementation, and analysis.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.