Introduction
Turner syndrome (TS) occurs in 1 in 2500 births and involves the complete or partial loss of one X chromosome (deletions, translocations, inversions, isochromosomes, and mosaicisms)1. TS is characterised by hypergonadotropic hypogonadism. Affected women are usually born with a normal number of primordial follicles that undergo accelerated atresia2. The majority of cases have primary amenorrhea3. Some women with primary amenorrhea, especially those with Y material in the karyotype, may have streak gonads4. They may have phenotypic characteristics and medical conditions including short stature, lymphedema, webbed neck, visual impairment, strabismus, otitis media, high arched palate, wide-spaced nipples, shield chest, multiple nevi, cubitus valgus, a short fourth metacarpal, ovarian dysfunction, hypertension, impaired glucose tolerance, as well as cardiac (coarctation or aortic anomalies) and renal abnormalities5, 6. Infertility is one of the most common challenges faced by TS patients6, 7. Fertility treatment options such as oocyte donation (OD) and embryo donation have made it possible for many women with TS to become pregnant8. Pregnancy in TS is associated with increased maternal and fetal morbidity9, mainly due to serious cardiac events, including aortic dissection in 2%10, 11.
Hagman et al. reported on the obstetric and neonatal outcomes after OD in 106 women with TS. Of 122 deliveries, 113 were singleton and 9 were twin deliveries. The multiple birth rate was 7.4%9. We here describe the case of a TS patient with three vaginal deliveries; to our knowledge, this is the only such case reported.
Case
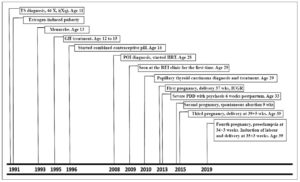
A 39-year-old woman, G4 P3 SA1, BMI 20.8 (height 152 cm, weight 50 kg), was diagnosed with TS at the age of 11 years, after showing poor growth (height less than the 5th percentile) and primary amenorrhea (Figure 1). Karyotype showed 46 X, i(Xq), isochromic of the X long arm. She was monosomic for the short arm of X and trisomic for the long arm (q), consistent with a type of TS. There was no family history of TS, congenital heart disease, sudden death, pacemaker implantation, stroke, diabetes, hypertension or hypercholesterolemia.
Upon TS diagnosis, growth hormone (GH) was given from the ages of 12 to 15 years. Puberty induction was initiated using estrogen alone for 1 year followed by estrogen and cyclic progesterone for 3 years. She achieved menarche at age 13 years, and subsequently switched to a combined contraceptive pill. She was seen at the premature ovarian insufficiency (POI) clinic at Mount Sinai Hospital, Toronto, Canada, and was started on transdermal hormonal replacement therapy (HRT) at the age of 28 years. Further investigations revealed normal kidneys, no signs of osteoporosis or diabetes, a tricuspid normal aortic valve with no stenosis or insufficiency, and normal-size aortic sinuses and ascending aorta, with no evidence of coarctation, confirmed by echocardiography and cardiac MRI. At age 29 years, she (and her husband) consulted the Department of Reproductive Endocrinology and Infertility (REI) at the same hospital with a view to planning a pregnancy. The patient was healthy without evidence of congenital heart disease. No abnormalities were found on endocrine function, renal and glucose tolerance tests.
Given the unremarkable pre-pregnancy screening, she was offered OD. However, in June 2010, before OD and in vitro fertilization (IVF) could take place, she was diagnosed with multifocal papillary thyroid carcinoma. A total thyroidectomy and bilateral central neck dissection with parathyroid auto-transplant was performed in August 2010. Her final pathology demonstrated metastatic disease and the patient underwent a repeat left lateral neck dissection in October 2010, and adjuvant radioactive iodine treatment in November 2010. A new nodule was seen on follow-up ultrasound in March 2011, for which she underwent right neck dissection. Histopathology reported this as a reactive and benign lymph node. She took levothyroxine thereafter, including during her pregnancies.
Our patient was seen at the REI department of Mount Sinai Hospital in 2013. Donor eggs were fertilized with her husband’s sperm and 11 embryos were obtained and frozen. She conceived four times with OD, IVF and embryo transfer, and achieved three successful vaginal deliveries. One pregnancy ended in spontaneous abortion. er pregnancies were closely followed by the Special Pregnancy Program at Mount Sinai Hospital.
Obstetric history
The first pregnancy, confirmed in September 2013, was complicated by late-onset intrauterine growth restriction (IUGR), diagnosed at 36 weeks. Induction of labor was started at 37 weeks of gestational age, with epidural coverage, and she delivered a 2,240 g (third percentile) female infant. Delivery was facilitated by episiotomy. The third stage was complicated by a retained placenta, which was manually removed in the labour and delivery room and did not require OR admission. The infant was admitted to the NICU after apneic episodes and remained there for four weeks. A small ventricular septal defect and a fenestrated atrial septal defect were diagnosed during NICU investigations. The infant was eventually weaned from oxygen in stable condition after 19 days in hospital.
At 6 weeks postpartum, the patient was diagnosed with postpartum depression (PPD) with psychosis that required admission due to thoughts of harming the baby and suicidal ideation. Initial treatment involved close observation, together with administration of the selective serotonin reuptake inhibitor escitalopram, which was titrated to 15 mg. Given the possibility of psychosis, quetiapine was also initiated and titrated up to 150 mg. Despite these treatments, the patient had ongoing suicidal and homicidal ideations and electroconvulsive therapy (ECT) was administered. She received six bilateral ECT treatments and improved steadily. After the course of ECT, she was started on hormone therapy in the form of estradiol gel, four pumps per day; normal physiological levels of serum estradiol were quickly achieved. The quetiapine was then weaned due to postural hypotension, but her affect remained bright. She was discharged in stable condition after 4 weeks in hospital and was continued on escitalopram and estrogel and sequential progesterone. Nursing occurred without problems.
The patient’s second pregnancy began in March 2015 but a spontaneous abortion occurred at 9 weeks of gestation. This required a D&C to evacuate the uterus.
The patient conceived again in 2015. In February 2016, at 39+5 weeks of gestation, she was induced with manual sweep of the membranes and then admitted to labour and delivery, where artificial rupture of membranes was done, and an epidural given. She delivered vaginally a male infant weighting 2,721 g. Serial assessment of aortic size by echo before the pregnancy and after delivery showed an ascending aortic size of 26 mm inner edge to inner edge, and 27 mm leading edge to leading edge towards the end of the pregnancy. On March 31, 2016 (postpartum) it was reported to be stable at 27 mm. The postpartum course was uneventful. High-dose HRT was initiated immediately postpartum to prevent recurrence of the postpartum psychosis, and cipralex was given prophylactically upon the advice of the perinatal psychiatry team. She remained well mentally.
In anticipation of a fourth pregnancy, a preconception consultation took place in February 2019 following MRI of the patient’s aorta in January 2019. Aortic MRI reported no significant change from the previous examination.
Her fourth pregnancy, at age 39, was complicated by pre-eclampsia at 34+3 weeks. She was initially treated with labetalol 100 mg twice per day, but she was admitted 3 days later and managed expectantly on the antenatal unit for about 48 hours, during which time labetalol dosing was increased to 900 mg/day. Her blood pressure remained high, in the presence of elevated liver function tests and proteinuria. The decision was taken to induce labour, and a successful and uneventful vaginal delivery occurred, at 35+3 weeks, in November 2019. Blood pressure was initially low postpartum but rose again at 48 hours postpartum. Discharge was delayed for an additional 2 days during which long-acting nifedipine was added and labetalol maintained. Subsequent to discharge her blood pressure stayed within normal limits. Nifedipine was discontinued after a few days postpartum, and then labetalol was progressively titrated downward. The newborn was fine both at the postpartum review and the 4-week review.
Discussion
We report the case of a patient with TS who was managed by a multidisciplinary team and was able to achieve four pregnancies and three successful vaginal deliveries. Our patient was diagnosed with TS at the age of 11 years due to her short stature and amenorrhea. She received appropriate medical intervention, including HRT, which was initiated during childhood. Appropriate timing of the start of HRT leads to acquisition of bone mass and an adequate uterine volume for pregnancy12. In TS patients who receive OD, the incidences of IUGR, preterm birth and birth defects have mostly been comparable to those recorded with conventional IVF13.
Pregnancy rates after embryo transfer with donated oocytes in women with TS are the same as or similar to those observed in other groups of oocyte recipients16. Nevertheless, pregnancies achieved with donated oocytes are associated with a high incidence of first-trimester bleeding and about a three-fold higher risk of gestation hypertension and pre-eclampsia when compared with standard IVF17. Multidisciplinary assessment and management of these complications is crucial.
Potentially life-threatening complications in patients with TS occur in 3.3% of pregnancies9, and include aortic dissection/rupture with a mortality rate of 2% 11. Therefore, in women with TS who are considering pregnancy, screening for cardiovascular disease using echocardiography and thoracoabdominal MRI before pregnancy is of fundamental importance. Women with TS have an elevated risk of developing impaired glucose tolerance, thyroid disease and hypertension6; hence, they are more prone to gestational diabetes mellitus, hypertensive disorders of pregnancy and preeclampsia18.
Multiple successful vaginal delieveries are possible in TS patients, when managed by a multidisciplinary team. Some reports indicate a higher proportion of caesarean section (CS) deliveries in women with TS compared with the general population. In a report by Hagman et al., of 122 pregnancies achieved by OD, 100 (82.0%) were delivered by CS. The major reasons for CS were cephalopelvic disproportion (CPD) and breech presentation9.
In accordance with the current TS clinical practice guidelines, GH should be started early (around 4–6 years of age, and preferably before 12–13 years) when there is evidence of growth failure 19. Until now, the use of GH in TS patients has not proven to increase the risk of papillary thyroid cancer. Nevertheless, there are 3 previous case reports of TS patients treated with GH who developed papillary thyroid cancer 20, 21. This raises the question of prospectively assessing the prevalence of papillary thyroid cancer in TS patients treated with GH in clinics with a larger volume of patients seen on a routine basis.
Awareness and prompt management of the postpartum challenges in TS patients are crucial. Women with TS are at a high risk of developing osteoporosis22, and in the postpartum period lactation enhances bone resorption, decreasing bone mass23. Women with TS may undergo a bone mass density evaluation during lactation, and appropriate treatment should be considered if needed. Increased depression and anxiety in TS have been related to hormone deficiency24. After her first delivery, our patient developed severe PPD with psychosis requiring a 4-week intensive inpatient treatment. It has been hypothesized that an estrogen-depleted state secondary to POI and lactation may be more pronounced during the postpartum when hormone levels dramatically decrease. Close monitoring and early replacement of depleted hormone levels in TS patients is recommended25.
Conclusion
Turner syndrome patients are able to successfully conceive through donor egg IVF cycles or donated embryos. Provided there is no significant cardiovascular disease, multiple vaginal deliveries are possible in TS women. Major complications, with potential life-threatening events, are not uncommon during prenatal and postpartum care in this population. A multidisciplinary approach, thorough preconception assessment, as well as antenatal and postpartum surveillance, should be offered to TS patients to improve pregnancy outcomes and minimize postpartum complications.
Conflict of interest statement:
The authors of this article have nothing to disclose.