Introduction
Anorexia nervosa (AN) is a serious eating disorder. A distorted self-perception of body image and an excessive fear of gaining weight result in restrictive eating habits and extreme underweight. On top of decreased body mass index (BMI), a number of other criteria are used to establish a diagnosis of AN [1]. The prevalence of this clinical condition is around 0.7–2.2% in adolescents and young women in the Western world, and the incidence in men is rising too [2]. AN manifestations include not only significant weight loss, but also a variety of other psychological disturbances and metabolic abnormalities. Furthermore, a significant percentage of women with AN develop menstrual disorders, such as oligo- or amenorrhea [3].
So far, it is not fully understood what factors might predict the presence of menstrual disorders in women with AN. As the link between adequate adipose tissue deposits and regular function of the gonadal axis is well known [3, 4], this question is especially important for those patients with the atypical type of the disease, that is with a relatively higher BMI [1]. The duration and quantity of menses is physiologically affected by a woman’s coagulation status. However, while this link is clear for heavy menstrual bleeding, scarce data exist for oligo-amenorrhea. To date, studies investigating the hematological profile of women with AN are very limited and hemostatic changes in AN have not been well described. Severe AN has been associated with pancytopenia and decreased bone marrow cellularity, which can cause mild thrombocytopenia. Furthermore, platelet hyperaggregability has been recognized and has been attributed, at least in part, to increased adrenoceptor density [5]. Some of the described hemostatic changes are related to acquired modifications probably linked to prolonged reduction in energy availability, but certain genes important for the coagulation process may also be implicated.
The aim of this study was to investigate the association between the genetic background of coagulation factors and the presence of oligo- or amenorrhea in adolescent girls diagnosed with AN compared with controls.
Methods
Study sample
This pilot study was conducted in a population of 40 young girls (14–17 years old) recruited from the Child and Adolescent Gynecology Outpatient Clinic of the 2nd Department of Obstetrics and Gynecology, Aretaieio Hospital, Athens, Greece. All participants were diagnosed with AN according to the American Psychiatry Association criteria (DSM-V, 2013) [1]. Inclusion criteria included body mass index (BMI) of 12.5–18.5 kg/m2, corresponding to values below the 5th percentile for age. Exclusion criteria included the presence of current or previously diagnosed metabolic bone disease, use of hormone supplements or contraceptives, and malnutrition due to other causes or current smoking. Moreover, we also recruited a total of 10 age-matched girls with normal menstruation and with no previous diagnosis of eating disorders, who served as controls. All the participants, as well as their legal guardians, signed an informed consent document and the study was approved by the Ethics Committee of Aretaieio Hospital.
Protocol study procedures
A detailed medical history was obtained from all the participants at the time of their first visit to the clinic. Moreover, all the girls completed questionnaires on demographic and socioeconomic characteristics, including questions regarding self-esteem in relation to their body. Weight and height were measured using electronic scales and a wall height meter, during early morning hours, with the participants dressed in light clothing. BMI was calculated using the formula BMI = body weight (kg) / height2 (m). The subjects’ age-related BMI z-score was calculated using the online calculator of the Children’s Nutrition Research Center, Baylor College of Medicine [6].
Fasting blood samples were collected in the morning, between 08:30 am and 09:30 am. Blood specimens used to assess polymorphisms were collected in K3EDTA (3mL) and stored at -20C; while those used to assess estradiol (E2) levels were centrifuged, and the serum was stored at -80C until assessment. Blood samples were collected during the 3rd–5th day of the menstrual cycle, in the case of participants with normal menstruation, and randomly in the case of those with amenorrhea. Serum levels of E2 were determined using standard laboratory techniques.
DNA preparation and pyrosequencing
DNA was extracted using the Nucleospin Blood QuickPure kit (Macherey-Nagel GmbH & Co, Duren, Germany), and single-nucleotide polymorphisms were detected by pyrosequencing. The primers used are shown in the Supplementary table. Polymerase chain reaction was carried out in 50mL reactions with 2mL DNA, 1mL of each primer, and 25mL Hot Start Master Mix (GE Healthcare Biosciences, Pittsburgh, PA, USA). After the initial denaturation at 95C for 5min, amplification consisted of 30 cycles of denaturation at 95C for 30s, annealing at 58C for 30s, and elongation at 72C for 30s, followed by a final elongation step at 72C for 4min. Reactions were carried out in microplates using the PyroMark Q24 system (Qiagen GmbH Hilden, Germany), and results were analyzed with the PyroMark Q24 software. The following polymorphisms were analyzed: MTHFR gene (MTHFR C677T; MTHFR A1298C), Glycoprotein IIIa receptor (Leu33/Pro), Prothrombin gene mutation (G20210A).
Statistical analysis
Statistical analysis was performed using SPSS v.25 (SPSS Inc., Chicago, IL, USA). The Chi-square test was used to test for the Hardy-Weinberg equilibrium in each of the evaluated polymorphisms. All the polymorphisms were evaluated comparing individuals carrying at least one mutated variant (homozygous or heterozygous) vs girls with the wild-type genotype. Qualitative variables were expressed as absolute frequencies (%) and quantitative variables were expressed as mean values and standard deviation (mean±SD) or median values and interquartile range. Differences between qualitative variables were assessed using the Chi-square test, whereas quantitative variables were compared using Student’s t-test for independent variables. We used Chi-square analysis to evaluate potential differences, between cases and controls, in the prevalence of at least one mutated allele of the assessed polymorphisms. Subsequently, we evaluated potential differences in the prevalence of genetic polymorphisms between girls with oligo- or amenorrhea and controls.
Logistic regression analysis models were fitted to evaluate the potential association between genetic polymorphisms proven to differ either between cases and controls or according to the presence of oligo-/amenorrhea. The models included each of the genetic polymorphisms in a one-by-one fashion, while we further adjusted for age, BMI z-score and estrogen levels. Statistical significance was set at the level of p <0.05.
Results
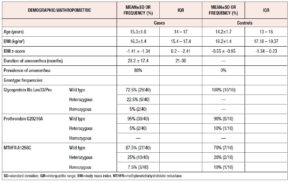
Table 1 presents the results of the descriptive analysis of the 40 girls with AN and the 10 healthy controls. As expected, the cases had significantly lower BMI and BMI z-score values than the controls. Moreover, amenorrhea was highly frequent in the girls with AN.
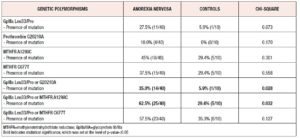
Table 2 shows the differences, between cases and controls, in the prevalence of the assessed genetic polymorphisms, and of their combinations. Accordingly, we observed that presence of GpIIIa Leu33/Pro mutation in at least one allelic variant exhibits a trend towards significance between girls with AN and controls (Chi-square p-value=0.073). Moreover, the presence of any mutation from the panel consisting of the GpIIIa Leu33/Pro mutation or the G20210A mutation of the prothrombin gene, was significantly more frequent in cases vs controls (35.0% vs 5.9%, p-value 0.028, Chi-square analysis). Finally, the presence of any mutation from the panel consisting of the GpIIIa Leu33/Pro mutation or the MTHFR A1298C mutation was significantly more frequent in cases compared with controls (62.5% vs 29.4%, p-value = 0.032, Chi-square analysis).
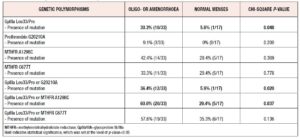
Table 3 presents the results of the comparison of the prevalence of the assessed genetic polymorphisms, or their combinations, between girls with oligo- or amenorrhea and controls. We observed that girls with oligo- or amenorrhea had a significantly higher prevalence of GpIIIa Leu33/Pro mutation in at least one polymorphic variant (cases vs controls: 30.3% vs 5.9%, p-value = 0.048, Chi-square p-value). In addition, the presence of any mutation from the panel consisting of the GpIIIa Leu33/Pro polymorphism and the G20210A polymorphism of the prothrombin gene was more commonly observed in girls with oligo- or amenorrhea vs girls with controls (oligo- or amenorrhoea vs controls: 36.4% vs 5.9%, p-value=0.020, Chi-square). Finally, the presence of any mutation from the panel, consisting of the GpIIIa Leu33/Pro genetic polymorphisms or the MTHFR A1298C genetic mutation, is more common in girls with oligo- or amenorrhoea vs girls with normal menses (oligo- or amenorrhoea vs controls: 60.6% vs 29.4%, p-value=0.037, Chi-square analysis).
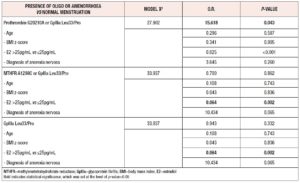
Logistic regression multivariate models were fitted to evaluate the association between single genetic mutations, or genetic panels, shown in the univariate analysis to be significant with respect to the development of amenorrhea in girls with AN vs controls. The models included the presence of oligo- or amenorrhea as dependent variable, while the genetic polymorphisms were included as independent variables in a one-by-one fashion. All models were adjusted for age, BMI z-score, levels of estradiol and diagnosis of anorexia nervosa. As presented in Table 4, we observed that presence of any mutation from the panel consisting of the G20210A or GpIIIa Leu33/Pro polymorphisms as well as estrogen levels, predict the development of amenorrhea (O.R. 15.618, p-value=0.043) in a model adjusted for age, BMI z-score and diagnosis of anorexia nervosa.
Discussion
This pilot study provided evidence that the presence of any polymorphism from the assessed genetic panel, relevant to coagulation factors, is more frequent in patients with AN than in controls, while adolescents with AN and oligo- or amenorrhea have a significantly higher prevalence of GpIIIa Leu33/Pro mutation in at least one polymorphic variant. Moreover, regression analysis showed that the presence of amenorrhea is associated with the GpIIIa gene polymorphism, as well as with estrogen levels.
The known possible predictive factors of oligo- or amenorrhea in women with AN are few. Adequate adipose tissue deposits are essential in order to ensure regular function of the gonadal axis, as a possible future pregnancy requires energy [3, 4]. Therefore, the presentation of amenorrhea is better understood in patients with the typical disease, that is with very low BMI [1]. In these cases, the limited body fat is not able to secrete adequate levels of adipokines, such as leptin, which act as signals for the hypothalamus [1]. Interestingly, amenorrhea has been restored by exogenous administration of leptin in women with hypothalamic amenorrhea [4]. However, the question of what factors predispose to menstrual disorders in women with the atypical type of AN, that is with a relatively higher BMI, remains very important [1]. Beyond hormonal profile, characteristics of menses, such as the type of bleeding, are physiologically affected by the coagulation status of a woman. As mentioned, this link is clear for heavy menstrual bleeding, while scarce data exist for oligo-amenorrhea. In this context, there may be an association with certain polymorphisms of genes implicated in the coagulation process. The results of this pilot study confirmed this general but sound pathophysiological hypothesis.
To date, studies investigating the hematological profile of women with AN are very limited and hemostatic changes in AN have not been well described. When 67 patients with AN were compared with 67 matched controls, it was found that those with AN had thrombocytopenia (<150x109/L; 10 vs 0%, p=0.03). Many of these patients also presented decreased bone marrow cellularity and abnormal architecture [7]. In another small study of seven young women with AN, plasminogen activator levels were significantly higher and the mean fibrinogen concentration was lower in patients compared with healthy controls [8]. Furthermore, platelet hyperaggregability has been recognized and has been attributed, at least in part, to increased adrenoceptor density in other (still few in total) studies [9-11]. Interestingly, most of these changes were reversible after proper supplementation and dietary guidance [5]. The distinction between acquired hematological changes, related to increased cardiovascular risk, and genetic defects remains important.
The strengths of this study are the homogeneity of our cohort and the inclusion of a group of age-matched controls. To our knowledge, this is the first study focusing on the genetic background of coagulation factors in women with AN in general, and specifically in AN associated with oligo- or amenorrhea. In the near future, when genetic tests are a reality in everyday clinical practice, the results herein reported, if confirmed, will render the diagnostic procedure and the therapeutic approach to these patients much easier and more precise. A limitation of the study is the small sample; however, this is only a pilot study and we aim to recruit more patients and controls. Another limitation may be the fact that the patients were recruited from a referral center, as this suggests that the results described may not reflect the general population of young women with anorexia nervosa.
In conclusion, this pilot study showed that the presence of oligo- or amenorrhea in girls diagnosed with AN is associated with low estrogen levels but also with a genetic background related to disorders of coagulation. Larger future studies are needed to confirm these findings.
Declaration of interests:
The authors do not report any conflict of interest