Introduction
Androgens in the female reproductive system
In women of reproductive age, dehydroepiandrosterone sulfate (DHEAS), dehydroepiandrosterone (DHEA), androstenedione, testosterone (T) and dihydrotestosterone (DHT) are found in the circulation, but only testosterone and DHT are able to activate androgen receptors. DHEAS, DHEA and androstenedione are considered prohormones, requiring conversion into T or DHT to express their androgenic effects [1] .
Testosterone
Testosterone is a steroid hormone (C19H28O2), and women have lower levels than men. The importance of estrogen and progesterone in female reproduction and chronic disease is well documented but the role of T in women’s health is less emphasized.[2] In females, serum androgen levels are higher than serum estrogen levels, even though the main effects of these hormones in women are those mediated by estrogen. It is reasonable to assume that T, too, has important physiological effects in women[3, 4] .
Research on androgens in women has focused almost exclusively on issues of excess, and it was recently shown that androgens play an important role in the health and well-being of women[5]. Testosterone is known to maintain muscle mass and bone strength, enhance sex drive, and facilitate an overall sense of well-being and zest for life in women. Yet the effects of T and its action on inflammatory conditions like asthma are not clear. Much of what is known comes from the study of conditions of excess or insufficiency in men and women, or from extrapolation of results from in vitro and animal studies [3].
Production of testosterone in females
In females, approximately 0.02 micromoles of T is secreted per day from the theca cells of the ovaries and the adrenal cortex [6]. Peripheral conversion of androstenedione (prohormone) in adipose tissue accounts for about half of the circulating T [7]. From birth to puberty there is no significant change in the level of T in females[8]. After puberty, increased peripheral conversion and increased production leads to higher T levels [9, 10]. Ovarian synthesis of testosterone occurs in theca cells and is mainly regulated by luteinizing hormone (LH) [11] and also by insulin[12]. Testosterone is then transported to the preovulatory granulosa cells, where it is aromatized to estrone and estradiol by 17-β-hydroxysteroid dehydrogenase type I, stimulated by Follicle stimulating hormone (FSH) [13]. Ovarian androgen production and conversion of T into estradiol are essential to the physiological process of ovulation and insufficient androgen production in the follicular phase can lead to anovulation [14] .
Measurements of serum testosterone
In females only 1% of T remains free and the rest is bound to proteins like sex hormone binding globulin (SHBG), albumin and cortisol binding globulin, reducing its bio-availability [7]. In ascertaining serum T levels, it is necessary to measure both the free testosterone (FT) and the albumin bound fractions. The ‘free testosterone index’ (FT level/SHBG level) is used for FT measurement in females[15]. The serum total testosterone (TT) level exhibits a circadian rhythm, with the highest observed in the early morning and the lowest in the late evening [16, 17]. Hence, serum T levels need to be interpreted with caution.
Total testosterone can be measured through immunoassays (IA) or by mass spectrometry (MS), which is considered the gold standard [18, 19]. Immunoassays can be performed either directly by radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), fluorescent immune assay (FIA), chemiluminescence immunoassay (CLIA), electro chemiluminescence immunoassay (ECLIA) or by means of each of these coupled with extraction and/or chromatography [18-20]. The extraction and/or chromatography procedures remove interfering proteins and increase measurement sensitivity[18]. Krakowsky et al. showed higher levels of T on ECLIA compared with liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method, which is probably the most appropriate for measuring low testosterone levels[21]. FT can be measured indirectly by equilibrium dialysis (EqD), directly by using an analog-based RIA (analog FT), or through standardized calculations. Equilibrium dialysis is costly but remains the gold standard in measuring FT[18].
Most current methods do not measure T levels in women accurately [22]. Measuring serum T levels has limitations due to issues with the method accuracy, precision, sensitivity and specificity at the low hormone levels found in women. Understanding of the clinical significance of T across the menstrual cycle (MC) has remained limited, partly due to these factors. Body mass index (BMI) [23], female smoking, alcohol consumption and exercise influence T levels in females [24].
Testosterone and chronic disease
In recent years, the role of T in the pathogenesis of asthma, rheumatoid arthritis, systemic lupus erythematosus (SLE), endometriosis, systemic sclerosis, and multiple sclerosis in women has been identified [25-32]. Indeed, low T has been linked to the pathogenesis of asthma [25], SLE [28], rheumatoid arthritis [30], and type 2 diabetes[33]. Testosterone supplementation has been found to be useful in chronic heart failure and to improve insulin resistance in elderly women [34]. The anti-inflammatory properties of T are explored as a probable disease-modulating mechanism in the above chronic inflammatory diseases. Studies demonstrate that T reduces TNF-α, interleukin isoforms, and C reactive protein (CRP), by various and often overlapping physiological mechanisms [35].
With the aim of establishing the current level of knowledge in this field, an in-depth critical analysis of the published literature was conducted to assess the variations in T levels throughout the MC.
Methodology
The authors performed an integrative systematic review of published literature, indexed in the PubMed database, describing changes in T levels during the MC in otherwise healthy females.
Database search
Studies reporting fluctuations in serum T levels during the MC were identified using the PubMed database (U.S. National Library of Medicine). The search included all relevant articles published up to October 2020. The PubMed database was searched using the terms ‘testosterone’ OR ‘androgens’ AND ‘menstrual cycle’ OR ‘ovarian cycle’ in a ‘title’, ‘abstract’ or ‘keywords’ search. Full articles of the included studies were accessed through the University of Sri Jayewardenepura.
Search method
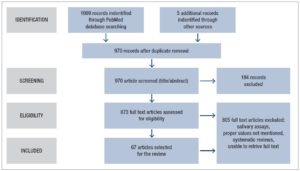
Articles identified from the keyword search were transferred to an Endnote database. Once duplicates were removed, the title, keywords and abstracts were reviewed for suitability for inclusion. All full texts except for 18 were accessed and reviewed for suitability (considering gender and age group of samples, and reporting of T levels). Forward citations of the studies retrieved were also traced and screened for possible inclusion and five additional articles were identified for inclusion. The search was conducted independently by KA and LA and the articles to be included in the review were determined through an interactive consensus process involving KA, NS, LA and DMSF (Fig.1).
Inclusion and exclusion criteria
For ease of comparability, the review included only research articles published in English and referring to serum samples, even though sputum or salivary T levels are considered good surrogates for serum T. Conference proceedings, editorials, commentaries, book chapters and book reviews were excluded. Studies describing two or more serum T readings across the MC were included even though such measurement was not the main objective of those selected studies. Studies that did not mention T values separately for each phase of the MC were excluded.
Data extraction
The full texts of the selected articles were reviewed and relevant variables were extracted into a datasheet. Extracted variables included, author/s, year of publication, method of detection of serum T, sample size, demographics of the sample, sample collection method, MC phase in which the sample was collected, and T values recorded.
Results
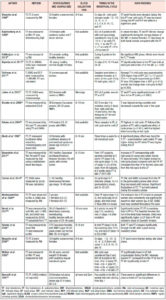
The primary search identified 1009 articles which were reviewed for duplicates, after which the title, keywords and abstracts of the remaining articles were reviewed. Five additional articles were identified by manually searching the reference lists and forward citations of included papers. A total of 67 articles were included in the review. Tables 1 and 2 summarize the studies included in the review.
Characteristics of studies
The included studies were conducted between years 1972 and 2020 and the number of participants ranged from 6 to 259. The studies concerned different ethnicities from North and South America, Europe, the Middle East, Asia and Australia. The females included were aged 18-45 years with a BMI ranging from 18 to 35 kg/m2. The T assessment methods varied between studies.
Timing in the menstrual cycle
Testosterone measurements were done at variable time points in the MC and few studies reported daily or every other day (EOD) serum sampling[36-39]. Some studies assessed T at four points in the MC i.e., early and late in the follicular phase (FP) and early and late in the luteal phase (LP) [40-42]; some at three points, i.e., early FP , mid cycle (close to ovulation) and LP [43-45]; and others at two points, i.e., in middle of the FP and the LP respectively [46-48].
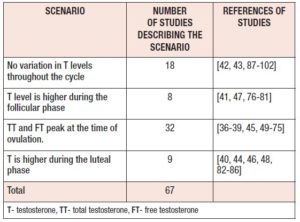
Multiple studies report that serum TT and FT levels peak during mid cycle at ovulation [36-39, 45, 49-75]. However, T level variation during the rest of the cycle does not seem to show a consistent pattern. Eight studies reported higher values during the FP[41, 47, 76-81], while nine studies reported higher values during the LP[40, 44, 46, 48, 82-86]. (Table 3). Many studies reported no change in T across the MC[42, 43, 87-102]. Out of the eighteen studies which did not observe any cyclical change, eight had assayed only two points (namely FP and LP) [89, 90, 92-95, 97, 98] and only four studies had sampled daily or EOD [87, 88, 96, 97].
Serum testosterone assessment technique
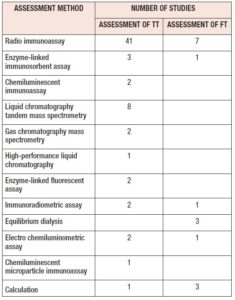
The included studies reported multiple methods of detecting serum T (Table 4). Studies which compared T detection methods, used more than one measurement method for T detection. Few studies assessed both TT and FT [37, 41, 42, 44, 47, 57, 68, 71, 78, 89], while the majority tested only TT [40, 43, 53, 61, 65, 70, 76, 79, 80, 83, 86, 91, 93].
Discussion
Testosterone peak at mid cycle
Most studies reported at least a minute mid-cycle increase in TT, which coincided with ovulation. Irrespective of the sample size and detection method, TT peaked during mid cycle in the studies where daily sampling was done. Studies which sampled T at two points during the MC did not observe a T peak as this could not be demonstrated by plotting only two measurements. Levels of T were lower in late LP and in the peri menstrual phase [61] and lowest close to menstruation consistently. Daily sampling is more accurate as the increased number of samples overcomes the diurnal variation and sampling bias.
The mid-cycle T increase is due to synthesis of T by the ovarian theca cells, mainly regulated by LH [10]. LH receptors are located on the theca cells during all stages of the MC. LH principally stimulates androstenedione production and, to a lesser degree, production of T in theca cells. Androstenedione is then transported to the granulosa cells where it is aromatized to estrone and then converted to estradiol by 17-β-hydroxysteroid dehydrogenase. In contrast, in the granulosa cells LH receptors only appear in mature follicles greater than 10 mm in diameter, i.e., in the antral follicle, which is most likely to develop as the dominant follicle[41, 103]. Ovarian androgen production and its conversion to estradiol are essential for follicle recruitment and the process of ovulation. Insufficient androgen production in the FP [14] and high androgen levels can lead to anovulation[104, 105]. Testosterone was significantly higher during the FP in females with anovulatory menstrual cycles [55].
Ovulation is triggered by the LH surge and it is plausible that a LH-mediated increase in ovarian androgen production occurs. The peak in T is unlikely to be due to adrenal androgen production or peripheral aromatization [69]. The mid-cycle androgen rise may accelerate follicular atresia and ensure that a single dominant follicle reaches the point of ovulation[68]. It is likely that androgens facilitate apoptosis and consequent follicle atresia only in mature antral follicles [104]. The mid-cycle increase in sexual activity could be attributed to the mid-cycle androgen spike[106, 107]. If high T is related to the LH surge at ovulation it must gradually recede from the FP to the LP and reported measurements showed both TT and FT to follow this pattern until the next FP [50, 60]. A reduction in SHBG is also reported in the mid cycle, coinciding with ovulation [45, 50] and possibly further increasing the FT levels, followed by an increase in SHBG levels during the LP [99].
The T rise in the mid cycle could affect smooth muscle and may assist ovulation and subsequent actions on the fallopian tube. The mechanism of action of T on smooth muscle and the inflammatory cascade needs to be studied further to understand the physiological basis for the rise in T during the MC.
The mid-cycle rise in FT was more prominent in younger females while it was not evident in older females aged between 43 and 47 years, who were cycling regularly and had normal levels of prolactin and thyroid-stimulating hormone [68]. The decreased concentrations of FT and androstenedione, without significant changes in SHBG, suggest that in older women these hormones are produced in lower quantities during the mid-cycle. It was concluded that this process was ovary dependent and not adrenal dependent as the observed changes varied with the stage of the MC.
Variation in reported mid-cycle testosterone values
Mid-cycle FT has been reported to be 63ng/dL (23-120ng/dL) [50] and as low as 23.72 ± 15.35 ng/dL [52]. However, most of the values reported in the included studies were within the currently accepted reference ranges (0.5-2.5 nmol/L or 15-75 ng/dL) [20]. The variability of reported serum FT values was dependent on several factors such as study design, study sample (age and BMI), assay techniques and their limitations, low T threshold and its statistical interpretation. The studies incorporated in the review mainly concerned apparently healthy females. Obesity, hypothyroidism, acromegaly, and diabetes mellitus, as well as glucocorticoids and anabolic steroids are known to decrease SHBG, resulting in low total T and high FT levels; SHBG levels are also known to decrease with old age, liver disease, and anticonvulsant and estrogen use, resulting in altered T profiles [18]. Higher levels of TT and FT with low SHBG have been reported in females with polycystic ovarian syndrome (PCOS) [108]. Studies involving women with chronic medical conditions, metabolic comorbidities like PCOS, diabetes mellitus, and other endocrine abnormalities are not within the scope of this review, and were therefore not included. However, BMI was seen to vary with different studies, with some studies also including females with higher BMI values. Of the limited number of studies which also evaluated overweight and obese females, some studies reported low TT [86], while others reported consistently high TT and FT values throughout the MC [41].
Variation of SHBG
An increase in SHBG in the LP compared with the FP was reported for ovulatory MCs [47, 54, 98, 109]. However, no consistent pattern of changes in SHBG, TT or FT levels was observed. Only a few studies had a sample size of more than 20 [42, 44-51, 77, 83], which could also affect these reported outcomes.
Timing of collection of samples during the menstrual cycle
As regards the definitions of the phases of the MC and the timing of sample collections, the studies showed considerable differences. When the FP was considered to last from day 0 to ovulation and the LP from ovulation to beginning of next menstrual cycle, there was uncertainty over the best day to sample in each of these two phases. Some studies, however, considered a range of days after the beginning of the MC for drawing blood in the FP, while mid cycle was defined as day 14-15 and the LP as after the mid cycle. The mid cycle was considered the late FP in some studies. In most studies at least three samples were taken, one during the FP, one at mid cycle, and one during the LP [43-45]. Instead, the few studies analyzing daily blood samples did not encounter the problem of determining ovulation and provided the best evidence for changing patterns of T [39, 62, 63, 100].
The selection of females
Most studies included females of reproductive age with regular menstrual cycles and they ranged from adolescents to premenopausal females. The participants also varied in terms of marital status and parity.
Although changes in T depend on whether a cycle is ovulatory or anovulatory, most of the studies did not identify MCs as ovulatory or anovulatory. Studies that did identify ovulatory and anovulatory cycles reported different patterns of T fluctuation, where in anovulatory cycles no mid-cycle rise in T was seen, with one study reporting higher T during the FP [56]. In contrast, in some studies no difference in T were seen between the females with ovulatory and anovulatory cycles [67].
BMI, smoking, alcohol consumption and exercise are known confounders for T levels in females [24]. However, very few studies have controlled for such confounding factors. Non-smoking females, without any chronic medical disease, who were not on any medication were studied in well planned studies omitting the errors due to the above factors [52, 78].
Sample timing during the day
It is important that timing of the sampling is uniform at all points of the MC as hormone levels are known to decrease after waking due to diurnal variation [110, 111]. In most studies sampling was done in the morning to get the highest T levels, and after an overnight fast. Fasting overnight was important as food interferes with the results of the analysis [112]. Some studies did sampling throughout the day and these provide the best evidence for the highest peak of T during the day.
Method of assessing testosterone
Most studies used the RIA method to measure TT, while some used the gold standard LC-MS/MS. Others used ELISA, CLIA, HPLC, ELFA. Older studies used RIA with organic extraction and chromatographic separation to remove interfering substances and matrix effects.[20] RIA has sensitivity to detect higher concentrations of hormone while errors may occur at lower concentrations especially in the late LP when hormone concentrations are lower. Recent studies have used automated platforms with nonradioactive methods like chemiluminescent detection. Liquid chromatography mass spectrometry (LC-MS) and LC-MS/MS can assess low concentrations of T seen in the MC [20].
It was difficult to compare studies as the assay techniques used and the T fraction tested (TT or FT) differed between studies; furthermore, outcomes were expressed in different forms, such as the T ratio or the FT index (calculated from TT and SHBG).
Future research on T changes during the MC needs to be designed to address the current knowledge gaps. MC phases and timing of blood sampling should be well defined. Future study protocols should include at least three sampling points per MC as a peak cannot be demonstrated when plotting only two measurements. Daily sampling, however, will overcome problems encountered in defining the phases of the MC and in detecting ovulation for mid-cycle sampling. The use of daily, morning samples, taken from healthy regularly menstruating females and tested using the liquid chromatography method, is recommended as the best option to achieve coherent results.
Conclusions
Both TT and FT peak at ovulation in females with regular (ovulatory) menstrual cycles. In the early LP, TT levels appear to be higher than in the early FP, but levels during the late LP seem to be lower than in the mid-follicular phase. Variations in serum T levels during different phases of the menstrual cycle seem to be related to whether the MC is ovulatory or anovulatory, to the T assessment technique used, and the timing of sample collection both during the MC and during the day. Further research should be conducted on larger populations and using more clearly defined criteria.
Conflict of interest
The authors declare that there is no conflict of interest.