Introduction
Exercise is one of the methods to prevent metabolic syndrome, which includes diabetes, hypertension and hyperlipidaemia. However, exercise has a higher risk for an increase of inflammation and production of reactive oxygen species. Exercise can also cause immune system impairments, muscle damage and an increased susceptibility to infection. According to Nieman (1993), athletes who conducted vigorous exercise tended to experience a higher risk for upper respiratory infection (URTI) [1], compared with individuals who either did not exercise, or who exercised moderately [2]. Moreover, vigorous exercise resulted in a suppression of multiple immune functions, and an increased incidence rate for upper respiratory infection, especially after exercise [3]. However, resent studies reported that the participants in the 86.5km ultramarathon found that salivary IgA levels 4-week before the race, and 2-week following the race were unrelated to the incidence of self-reported URTI [4], so acute exercise participation does not heighten risk of opportunistic infection especially individuals who regularly conducted vigorous exercise [5]. Additionally, reduction in the frequency and function of lymphocytes and other immune cells in peripheral blood in the hours following vigorous and prolonged exercise does not reflect immune suppression [5]. Taken together, athletes who conducted vigorous exercise regularly did not induce acute exercise-induced suppression of immune function, but most of studies targeted for men, and still unclear the response who have regular menstrual cycle.
The Natural killer (NK) cell is a component of the innate immune system. and serves to remove virally infected cells. Interleukin-2 (IL-2) is a cytokine that enhances the cytotoxic activity of NK cells, and prostaglandin E2 (PGE2) is one of the mediators that supresses NK cell activity. Previous study has reported that a vigorous intensity of aerobic exercise induced an increase in NK cell activity immediately after exercise, and that 2 hours after exercise, NK cell activity was dramatically decreased, concomitant with decreased levels of IL-2 and increased levels of PGE2 [6]. However, another study found contrasting results in which moderate and lower intensity aerobic exercise similarly resulted in an increase in NK cell activity and IL-2 levels immediately after exercise, but a significant decrease was not found 2 h after exercise [7]. Furthermore, higher levels of progesterone and oestradiol administration have been shown to suppress IL-2 receptor expression and reduce NK cell activity [8]. In contrast, physiologically lower oestradiol, or lower levels of oestradiol administration, suppressed the production of inflammatory cytokines, including interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), as well as PGE2 levels [9]. Thus, for different exercise intensities, the NK cell activity responses are different, and the effects of progesterone and oestradiol on innate immune function may differ depending on concentration. However, it remains unclear whether a single bout of vigorous aerobic exercise affects the immune function responses in women, especially for younger women who have a regular menstrual cycle.
Although a previous study reported that the immune function responses induced by aerobic exercise were similar for each sex, they found an increase in estradiol and a decrease in follicle stimulating hormone (FSH) induced enhancement of NK cell activity [10]. Moreover, NK cell activity was significantly lower at the luteal phase compared with the follicular phase of menstruation [11]. Other studies showed that oestradiol played a role to improve humoral immunity and impaired cellular immunity [12]. Additionally, studies have revealed a progesterone induced decrease in the cytotoxicity activity of killer T cells through suppression of differentiation into T helper cells (Th1) [13,14]. Thus, women have a greater variation of these hormones due to the menstrual cycle; however, the effect of the menstrual cycle on immune function and aerobic exercise induced responses in immune function especially for women who have menstrual cycles is not fully elucidated.
Thus, we hypothesized that a single bout of exercise affects the immune function responses differently between the follicular and luteal phases in healthy young women with normal menstruation. The purpose of this study was to investigate the effects of acute vigorous aerobic exercise on NK cell activity, PGE2, a mediator that suppresses NK cell activity, and IL-2, a cytokine that enhances NK cell activity, at both follicular and luteal phases in healthy young women.
Materials and methods
This study protocol was approved by the Human Research and Ethics Committee at Graduate School of Human Development and Environment, Kobe University. Participants provided informed written consent before commencement of the study, which were conformed to the standards set by the Declaration of Helsinki.
Participants
Eleven healthy women (age: 22±0.54) who have normal menstruation and normally active ( O2 peak: 36.65±1.55), participated in this study. Participants with abnormal menstruation, and those who were taking oral contraceptives, smoking, and taking medication (diabetes, hypertension and hyperlipidemia), dietary nutritional supplements and habitually conducted vigorous exercise were excluded from this study. The participants were recruited in our university by e-mail, and were examined by a physician to confirm that none had medical problems that might preclude participation or affect the results. The participants’ menstrual cycles were determined by measurement of their basal body temperature for 2 months, and by a questionnaire for menstrual cycle frequency according to previous studies [15]. The room temperature was maintained at 22-24 ℃ throughout all of the experiments.
Measurement of O2 peak
To determine participant fitness and to set the workload for subsequent exercise, all participants underwent an incremental exercise test to exhaustion with a cycle ergometer in a semi recumbent position (Standard Aerobike 828E; Monark, Sweden) at first day visit. The incremental exercise test began at an initial power output of 90 W, and the workload was increased by 15 W every minute until exhaustion. The pedalling rate was maintained at 60 revolutions per min (rpm) with the aid of metronome. Minute expired ventilation, oxygen uptake ( O2) and carbon dioxide output were recorded during the test and were averaged every 30 s afterward. The highest O2 value obtained during test was used as the peak O2 ( O2 peak). Respiratory variables were determined by an online system with breath-by-breath, according to our previous studies [16]. The participants breathed through a leak-free nasal mask (Free-Fit mask, Minato Medical Science CO., LTD. Osaka, Japan). Expired gas volume was measured (Aeromonitor AE-310S, Minato Medical Science CO., LTD). Breath-by-breath data were analysed continuously with customised computer software. The heart rate was continuously monitored (Polar, H10n heart rate sensor, Tokyo, Japan) during the experiments.
Exercise protocol
On the second day visit, aerobic exercise at 75% of the O2 peak was conducted using a cycle ergometer at both follicular, and third day visit also conducted same exercise at luteal phases. To normalise for circadian variations in the hormonal state, exercise took place in the morning within 4 h after waking. Additionally, to avoid the effects of nutrition and food consumption, all participants were instructed not to eat or drink after midnight, except for water, on the night before the experiments.
Blood sampling
Fasting blood samples were obtained to avoid the effects of food consumption, similar to our previous studies [16,17]. On arrival at the laboratory, a blood sample was taken from the fingertips and stored using the MBS Capillary (MBS, Tokyo, Japan), which included EDTA-2Na. Blood samples were taken before and immediately after exercise, and at 1h and 2h after the exercise session. Plasma samples were immediately centrifuged at 1500 g and 4 ℃ for 15 min. The supernatant was immediately transferred to polypropylene tubes and stored at -30 ℃ until analysis.
Sandwich enzyme immunoassay
The level of NKp30 (Xpress Bio, Fredrick, MD, USA), IL-2 (Proteintech Group, Inc., Rosemont, IL, USA) and PGE2 (Cayman Chemical, Ann Arbor, MI, USA) in the plasma was determined using a sandwich enzyme immunoassay kit. The immobilised polyclonal antibodies against NKp30, IL-2 and PGE2, were used, whereas the secondary horseradish-peroxidase-coupled antibodies were monoclonal. A microplate reader was used to measure the optical density at 420 and 450 nm (Thermo Fisher Scientific, Multiskan FC, Yokohama, Japan). All samples were assayed in duplicate.
Statistical analysis
All values were expressed as mean ± standard error (SE). Statistical evaluation was performed using two-way repeated analysis of variance (ANOVA) (group x time). When significant differences were observed, a post hoc comparison test was used to correct for multiple comparisons (Bonferroni test). For ANOVA, P < 0.05 was considered significant for post hoc tests. All tests were conducted with StatView version 5.0 (SAS Institute Inc., Tokyo, Japan).
Results
Physical characteristics
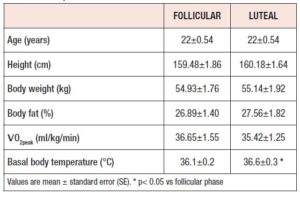
There were no significant differences in the physical characteristics between the follicular and luteal phases (Table 1). Additionally, there were no significant differences between follicular and luteal phases in body composition such as body weight and percent fat. The participants’ menstrual cycles were 30.58 ± 0.65 days; a normal menstrual cycle was confirmed according to the Japan Association of obstetricians and gynaecologists guidelines [18]. Additionally, basal body temperature was significantly higher at luteal phase than follicular phase (Table 1: P < 0.05).
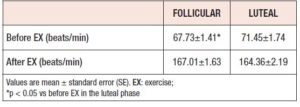
The participants’ heart rates before exercise were significantly lower in the follicular phase than in the luteal phase (P < 0.05), but no significant difference was noted for the heart rate after vigorous exercise between the follicular and luteal phases (Table 2).
NK cell activity (NKp30 level)
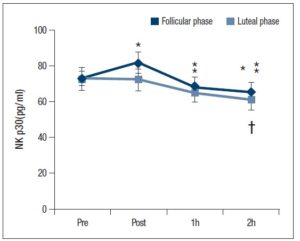
The NKp30 level tended to be higher (P = 0.056) after exercise at the follicular phase compared with the luteal phase. Further, the NKp30 level at the follicular phase was significantly lower 1 and 2 h after exercise compared with baseline (P < 0.05). At the luteal phase, the NKp30 level was significantly lower only at 2 h after exercise compared with before and immediately after exercise (Fig. 1:P < 0.05).
IL-2 and PGE2 levels
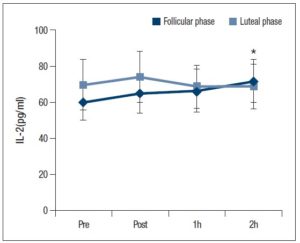
There was significant interaction between exercise time and the menstrual cycle for the IL-2 level (Fig. 2: P < 0.05). Also, a multiple comparison using the Bonferroni method revealed that the IL-2 level was significantly higher 2 h after exercise compared with the baseline for the follicular phase (Fig. 2: P < 0.05).
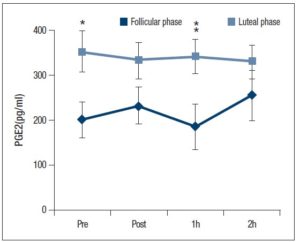
There was a significant main effect for the menstrual cycle and the PGE2 level (Fig. 3: P < 0.05); one-way ANOVA analysis found a significantly higher PGE2 level at the luteal phase baseline compared with the follicular phase (Fig. 3: P < 0.05). Although the PGE2 level was slightly decreased 1 h after exercise at the follicular phase, it was significantly higher and a higher level was maintained at the luteal phase (P < 0.05).
Discussion
In this study, at both menstrual phases, NK cell activity was significantly reduced after 1 and/or 2 h of acute vigorous aerobic exercise compared with baseline and immediately after exercise. On the other hand, the IL-2 level was significantly higher 2 h after exercise at the follicular phase, and a higher PGE2 level was maintained after exercise at the luteal phase compared with the follicular phase. Therefore, the responses of mediators and regulators of NK cell activity (IL-2 and PGE2) to acute vigorous aerobic exercise were different between the follicular and luteal phases.
Previous studies by Nieman et al. (1993) reported that NK cell activity, measured with flow cytometry, within 3.5 h after a single bout of high intensity aerobic exercise (80% O2 peak). Furthermore, Tvede et al. (1993) reported that NK cell activity was significantly decreased after 2 h of high intensity aerobic exercise (75% O2 peak). In the present study, the NKp30 level was measured to evaluate NK cell activity, which is conventionally measured with flow cytometry or radioisotopes [19]. NKp30 levels were significantly reduced after 1 and/or 2 h of acute vigorous aerobic exercise in both menstrual cycles. The implications of these observations are consistent with the aerobic exercise-induced decrease in NK cell activity observed in previous studies [1,6]. A study done by Yovel et al. focusing on the effect of the menstrual cycle on NK cell activity (at rest) in women found that NK cell activity did not differ between the follicular and luteal phases [20]. Consistent with previous studies, in the present study, NKp30 levels at baseline (at rest) did not differ among menstrual cycles. This was true even though NKp30 levels tended to be higher after vigorous aerobic exercise at the follicular phase compared with the luteal phase. One of the possible reasons for this finding was that the oestradiol level could have caused the enhancement of humoral immunity and the concomitant attenuation of cellular immunity [12]. Moreover, progesterone plays a role in attenuating the T cell differentiation into Th1 cells [13]. The experiments in the present study were conducted in the early follicular phase, which is associated with lower oestradiol levels compared to the later stage of the follicular phase. Subsequently, no significant differences in the NK cell activity were noted between the follicular or luteal phases for both oestradiol- and progesterone-induced attenuation of cellular immunity. However, oestradiol, progesterone, luteinizing hormone (LH) and FSH levels were not measured in the present study to determine the menstrual cycle phase. Using these hormones to determine the menstrual cycle phase would be required for further investigation.
Oestradiol and progesterone play a key role in regulating immune responses in women. Previous studies found no significant difference in IL-2 levels between the follicular and luteal phases at rest [21, 22]; however, the addition of 10-5 M to 10-4 M oestradiol into T cells caused a significant decrease in IL-2 receptor expression [8]. In studies performed on rodents, higher levels of oestradiol administration inhibited NK cell activity [23]. Additionally, oestradiol and progesterone has been shown to inhibit DNA synthesis, as well as cytotoxic T lymphocytes [24]. Furthermore, oestradiol mediates the reduction of IL-2 production, and the reduction in the suppression activity of splenocytes [25]. On the other hand, lower levels of progesterone administration inhibit IL-6 production, and treatment with physiological oestrogens resulted in a delay in spontaneous apoptosis and attenuated mRNA expression of inflammatory mediators [9]. Thus, effects of progesterone and oestradiol on innate immune cells may differ depending on their concentration. In the present study, plasma samples were taken during the early follicular phase when oestradiol levels were low. Further studies are needed to investigate the relationship between oestradiol, progesterone levels and immune function, especially at the later follicular phase where oestradiol levels are high. Accordingly, higher PGE2 levels were shown at the luteal phase compared with the follicular phase in this study. Moreover, it may be possible that the PGE2 level could be more volatile at the follicular phase if a large fraction of the oestradiol level had occurred at the follicular phase. Taken together, the IL-2 and PGE2 levels could be affected by the oestradiol level according to previous studies and this study.
There are some mediators and cytokines which regulate NK cell activity such as interferon-γ, and IL-12 that are also related to the activation of cytotoxic T cells for adaptive immunity. To attenuate adaptive immunity function, these cytokine and cytotoxic T cell activity levels will require examination in a future study. Moreover, other activation receptors for NK cell activity, such as NKp44 and NKp46, were also increased by the enhancement of NK cell activity according to previous study [26], and NK cell activity was enhanced by IL-2 through an increase in the activated receptor natural killer group 2D (NKG2D) [27]. Measurement of the activity of other receptors to attenuate NK cell activity in response to vigorous exercise at both luteal and follicular phases should be conducted. Cortisol is generally anti-inflammatory and plays a role in the containment of an immune response; however, chronic elevations lead to an accumulation of stress hormones and increased production of inflammatory cytokines, which further compromise the immune response [28]. Additionally, higher levels of cortisol administration have been shown to reduce LH pulse frequency in the follicular phase and block the resultant increase in oestradiol [29]. Therefore, chronic levels of cortisol influence the menstrual cycle, as well as immune function. However, the present study did not measure cortisol levels and it is suggested that future studies aim to include the effect of cortisol levels on immune function and other reproductive hormones.
Conclusion
In this study, the NK cell activity in response to vigorous aerobic exercise did not differ between the luteal and follicular phases. On the other hand, the responses of mediators and regulators of NK cell activity, IL-2 and PGE2, were changed by the menstrual cycle. Thus, different responses to vigorous aerobic exercise were shown for the NK cell regulated mediators and cytokines at the luteal and follicular phases of the menstrual cycle.