Introduction
Background
In recent years, endometrial cancer has been found to be the second most common gynecological malignancy in China, after cervical cancer [1]. Endometrial cancer is usually characterized by a thickened endometrium, found on transvaginal ultrasonography (TVS) in postmenopausal women with or without abnormal uterine bleeding [2]. Measurement of endometrial thickness (ET) using TVS is a simple and accurate method for estimating the risk of endometrial cancer [3]. ACOG Committee Opinion No. 734 recommends that the negative predictive value for endometrial cancer is greater than 99% with an ET of 4 mm or less detected by TVS in postmenopausal women with postmenopausal vaginal bleeding (PMB) [4]. PMB with or without endometrial thickening may occur as a result of other diseases besides endometrial cancer, such as endometrial polyp (EP), hyperplasia of the endometrium, submucous myoma, endometrial atrophy, endometritis, etc. [5]. But the cut‐off level of ET in postmenopausal women without PMB is still controversial and unclear for the purposes of malignancy diagnosis [6]. In such cases, evaluating women according to the above criteria, i.e. an endometrial measurement greater than 4 mm, may lead to unnecessary invasive procedures and place a mental, physical and financial burden on patients [4].
Objectives
The goal of this study was to explore the endometrial characteristics of postmenopausal women who visited Beijing Chuiyangliu Hospital, as well as the relationship between ET and endometrial diseases, to provide evidence to support the diagnosis and treatment of endometrial diseases in postmenopausal women. We also tried to identify a cut-off value of ET able to predict malignancies in asymptomatic postmenopausal women with ET ≥ 4 mm.
Material and methods
Study design and participants
This study is retrospective observational research. A total of 451 postmenopausal women who attended the Department of Gynecology at Beijing Chuiyangliu Hospital between June 2017 and June 2019 and met the inclusion criteria were included in the study.
Inclusion criteria: (1) menopause duration ≥1 year; (2) TVS showing endometrial thickness ≥ 4 mm with or without PMB; (3) all patients with endometrial abnormalities underwent hysteroscopic examination after routine evaluation in the gynecology clinic using transvaginal ultrasound. Endometrial biopsy or endometrial lesion removal was performed to identify endometrial pathology.
Exclusion criteria: (1) patients undergoing oral chemotherapy or menopause hormonal therapy; (2) patients with ovarian tumor, endometriosis or adenomyosis; (3) patients with other malignant tumor history; (4) comorbidities for which HS is not appropriate.
According to TVS measurements of ET and evidence of postmenopausal uterine bleeding, the 451 enrolled participants were divided into 3 groups, 21 in Group A: ET < 4 mm with PMB, 175 in Group B: ET ≥ 4 mm with PMB, 255 in Group C: ET ≥ 4 mm without PMB. All participants signed informed consent.
Ethics approval
This study was approved by the Ethics Committee of Beijing Chuiyangliu Hospital (Ethics approval number: 2021-012KY).
Assessment of endometrial thickness
Endometrial thickness was reported in millimeters (mm). TVS was performed as clinically indicated, and the results were interpreted by doctors specializing in pelvic ultrasound. As mentioned, in 2018, the American College of Obstetricians and Gynecologists (ACOG) proposed that the negative predictive value for endometrial cancer is greater than 99% with an ET of 4 mm or less detected by TVS in postmenopausal women with PMB [4]. The same guideline recommends either endometrial sampling or TVS for evaluation of women with an initial episode of PMB; if TVS is performed and the resulting ET measurement is ≤ 4 mm, endometrial sampling is not required. Therefore, in this study, ET ≥ 4 mm with or without uterine space occupation was used as the criterion to determine a positive TVS result.
Hysteroscopy evaluation
During the operation, an Olympus uterine cavity examination mirror (4.5 mm diameter) and a circulation perfusion electric resection mirror (9.0 mm diameter) were used. The procedure was performed under general anesthesia administered intravenously in strict compliance with the specifications of the HS operation and was performed by experienced doctors with over five years of experience in HS surgery. Injection of 0.9% sodium chloride was used as the distending medium in the examination procedures, while 0.5% mannitol was the distending medium used in the polypectomy procedure. The distension pressure was set at 80~100 mmHg (1 mmHg=0.133 kPa) and the flow rate was 260 ml/min. Surgical information and images can be recorded during HS. The cervical canal, the uterine horns on both sides of the uterine cavity, and the endometrium in the uterine cavity were comprehensively observed. All participants underwent diagnostic evaluation via endometrial sampling.
Pathology diagnoses
The final pathology diagnosis after endometrial sampling was considered the reference diagnosis. Pathology diagnoses were made clinically by gynecologic pathologists. Final endometrial pathology diagnoses from the baseline visit were classified as: Benign, including atrophic endometrium, EP, endometrial hyperplasia without atypia, endometritis, submucosal fibroids; or Endometrial intraepithelial neoplasia, including all diagnoses of hyperplasia with atypia, and endometrial cancer.
Statistical analysis
SPSS 23.0 statistical software was used for analysis. The normality of distribution was assessed with the one-sample Kolmogorov-Smirnov test. The quantitative variables, which were normally distributed, were presented as mean ± standard deviation (SD). Differences among three groups were analyzed by one-way analysis of variance (ANOVA) and the LSD-T test was used for multiple comparisons among the groups. The Chi-square test was used for categorical variables. We plotted the receiver-operating characteristic (ROC) curves with the true positive rate (sensitivity) on the y-axis and the false positive rate (1 – specificity) on the x-axis. We then calculated the area under the curve (AUC) and determined the optimal cutoff for ET in postmenopausal women without PMB based on Youden’s J index. P<0.05 was considered statistically significant.
Results
Clinical characteristics of the participants
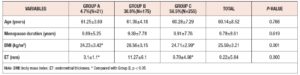
Based on the inclusion and exclusion criteria used in this study, a total of 451 women were enrolled and included in the data analysis: Group A made up 4.7% of the whole sample (21/451), Group B 38.8% (175/451), and Group C 56.5% (255/451). The women’s mean age was 60.14 ± 8.52 years, and the mean duration of menopause was 9.78 ± 8.61 years. No significant differences in these two variables were observed among the three groups (p>0.05). The mean ET in Group B was higher than that in Group A and Group C, and the mean body mass index in Group B was higher than that in Group A and Group C (p<0.05) (as shown in Table 1).
The characteristics of endometrial disease in the three groups
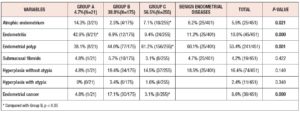
Table 2 shows the characteristics of the benign and malignant endometrial diseases found in the three groups. Statistically significant differences were observed between the three groups in the proportions of women with atrophic endometrium, endometritis, EP, and endometrial cancer (p<0.05). The percentage of EP in Group C was significantly higher than in Group B and Group A (p<0.05). There were 401 patients with benign endometrial diseases, with EP (60.1%, 25/401) being the most frequent of these endometrial lesions. Endometrial cancer occurred in 17.1% of cases in Group B, significantly higher than the percentage in Group C (p<0.05), and higher than that in Group A although the difference was not statistically significant (p=0.142). Group C was further divided into two parts, Group C1: ET is equal or greater than 4mm but less than 10mm (ET, ≥4<10 mm) without PMB; Group C2: ET≥10 mm without PMB. There were 154 cases in Group C1, including 1 case of endometrial cancer accounting for 0.6% (1/154); Group 2 had 101 cases, including 7 cases of endometrial cancer accounting for 6.9% (7/101). The proportion of endometrial cancer in Group C2 was significantly higher than that in Group C1 (p<0.05). Only 1 woman with endometrial cancer was detected in Group A; her body mass index was 34g/m2 and she had type II diabetes mellitus.
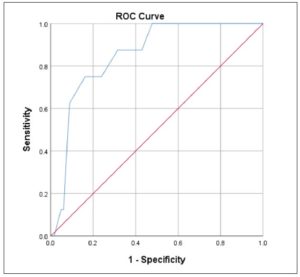
The ROC curve of ET was constructed to evaluate its predictive value for predicting malignant disease in postmenopausal women with ET ≥ 4 mm without PMB (Fig. 1).
In this study, there were 8 cases (3.1%) of endometrial cancer in Group C, in which the mean ET was 9.70±4.98 mm. The ROC curve for the diagnosis of endometrial cancer based on ET measured by TVS in Group C was plotted. The ROC curve for the diagnosis of endometrial cancer based on ET measured by TVS in Group C was plotted., Based on the ROC curve, when the ET was equal to 10.15 mm, the sensitivity for predicting a malignant diagnosis was 75.0%, the specificity was 86.2%, and the highest Youden index was 0.612 (AUC=0.852, 95%CI 0.752–0.951, p =0.001).
Discussion
The present study found that 91.4% of postmenopausal women had benign endometrial diseases, which is consistent with the relevant results from both domestic and foreign studies [7,8]. EP (241/451, 53.4%) accounted for the highest proportion of these endometrial lesions, also within two of the three groups of women (B, 44.0% and C, 61.2%). No statistical information exists on the prevalence of EP among postmenopausal women in China. This study was still unable to assess the prevalence of endometrial disease in postmenopausal women since the sample analysis was restricted to patients with PMB or women with abnormal ET detected on TVS. Despite these limitations, we still believe that this study has some significance. Endometrial polyp is a common benign disease of the uterus and can occur in females of any age, even though it has been shown to increase with increasing age [9]. Postmenopausal women with EP are at increased risk of malignancy compared with premenopausal women [10]. Reportedly, 10~34% of endometrial cancers in postmenopausal women have been associated with EP [11]. Sandra Bel et al. [12] reported that the risk of malignancy was 12.3% in patients aged over 59 presenting PMB with EP. For all other subgroups, the risk varied between 2.31 and 3.78%. Anna Uglietti et al. [13] reported that the frequency of malignancy among women with EP was associated with menopausal status, and with the presence of PMB. Endometrial serous carcinoma (ESC) is frequently associated with EP [14]. ESC is an aggressive tumor that has a high propensity to develop distant metastases even in the absence of myometrial invasion [10]. Accordingly, patient records should be reviewed on an individual basis, taking into account the patient’s preexisting conditions. Hysteroscopic resection of EP should therefore be routinely proposed in older postmenopausal women with PMB, after providing clear and appropriate information.
This study shows that the mean ET in Group C was 9.70±4.98 mm, significantly lower than that in Group B (11.27±6.1 mm). The proportion of endometrial cancer in Group B was significantly higher than that in Group C (p<0.05), but 1 case constituted 4.8% (1/21) of Group A, no significant difference was observed between Group B and Group A (p=0.142). Endometrial cancer is the most common gynecological malignancy in developed countries [4], and the second most common gynecological malignancy in China [1]. In patients with PMB, ETs measured by TVS were significantly correlated with the risk of endometrial cancer [7]. ACOG Committee Opinion No. 734 recommends that because rare cases of endometrial carcinoma (particularly type II) can present with an ET of less than 3 mm, persistent or recurrent uterine bleeding should prompt a histologic evaluation of the endometrium regardless of ET [4]. The present study reported 1 endometrial cancer case in Group A (ET < 4 mm and PMB), associated with a body mass index of 34kg/m2 and type II diabetes mellitus. Obesity and type II diabetes mellitus are independent risk factors for endometrial cancer. Clinical risk factors for endometrial cancer should also be taken into account when evaluating PMB, including, but not limited to, age, obesity, use of unopposed estrogen, specific medical comorbidities (e.g. polycystic ovary syndrome, type II diabetes mellitus, atypical glandular cells on cervical cytology), and family history of gynecologic malignancy [15]. A woman with a thin endometrium should undergo histological investigation if she experiences repeated episodes of PMB and ongoing PMB [15]. For diagnostic purposes, endometrial histopathology should be carried out more actively among women with high-risk factors.
Fear of endometrial cancer is the leading reason for outpatient medical checks in postmenopausal women with PMB or abnormal ET. In most cases of endometrial cancer, PMB is the most common symptom, experienced by 90% of women. However, depending on the sensitivity and specificity of the screening tools, up to 20% of women may be symptomless when diagnosed [16]. A standardized cutoff value for postmenopausal women without PMB that accurately differentiates normal endometria from pathologically thickened endometria has not been established [17]. For this reason, the majority of women are admitted to undergo invasive diagnostic testing. This may lead to patient anxiety and increased costs. The results of the present study gave us a ROC curve showing that when ET was 10.15 mm, sensitivity was 75.0%, specificity was 86.2%, and the highest Youden index was 0.612. Berna Seckin et al. [6] indicated that an ET of 8.2 mm is the best cutoff value for detecting malignancy in symptomatic women. Ahmed Ghoubara et al. [18] reported that utilization of a ≥10 mm ET threshold to prompt investigations did not miss any cases of endometrial atypical hyperplasia or cancer. The ACOG does not recommend routine endometrial biopsy in postmenopausal women with asymptomatic ET >4 mm [4]. According to the Society of Obstetrics and Gynecology of Canada (SOGC), a woman who has endometrial thickening and other positive findings on ultrasound, such as increased vascularity, inhomogeneity of endometrium, particulate fluid, or thickened endometrium over 11 mm, should be referred to a gynecologist for further investigations [19]. A systematic review and meta-analysis have suggested that asymptomatic women with risk factors, such as increasing age, diabetes, hypertension, obesity, nulliparity, chronic anovulation, and the use of unopposed estrogen, should be evaluated more rigorously [20].
In conclusion, EP is the most common endometrial lesion in postmenopausal women. ESC is frequently associated with EP. Further research is needed to determine the optimal ET threshold for endometrial cancer in postmenopausal women without PMB. Further diagnostic evaluation of the endometrium should be determined by the clinician on an individual patient basis, taking into account abnormal bleeding, risk factors for endometrial lesions (e.g., abnormal body mass index, medical comorbidities, family history), and patient preferences.
Acknowledgements
We thank all participants and staff at the study sites for their cooperation.
Author contributions
GZ, RJ, XR contributed to the conception and design of the study. GZ, HW contributed to the acquisition of data, and RJ to the analysis and interpretation of data. GZ, RJ drafted the article, and XR revised it critically. All authors read and approved the final manuscript.
Disclosure statement
The authors declare there is no conflict of interest associated with this manuscript.
Funding
This study was supported by the Beijing Chaoyang District Science and Technology Plan (CYSF1713), National Menopause Health Care Specialist Construction Unit of China [No. (2020)30)].