Introduction
Endometriosis is a disease defined by the presence of endometrial glands and stroma outside of the endometrial cavity, causing chronic inflammation [1]. Deep infiltrating endometriosis (DIE) is considered its most severe form, with lesions extending underneath the peritoneum and affecting pelvic organs – such as the bowel, the ureters and the bladder – and connective-fascial structures including the uterosacral ligaments (USLs) and the recto-vaginal septum [2]. DIE is often associated with severe painful symptoms, and may cause impairment of intestinal, urinary and sexual functions [1,2].
Among the disorders related to endometriosis, a marked impact on pelvic floor muscle (PFM) function has been described [3-5]. PFM dysfunction includes disturbances in muscle contraction, relaxation and coordination, such as spasm and hypertonia, sacral reflex abnormalities, abnormal anal and urethral sphincter tone, and tenderness at myofascial trigger points [3-8], which may contribute to the pathogenesis of endometriosis-associated symptoms, including chronic pelvic pain, dysfunction in micturition or defecation, and dyspareunia [9-12].
Ultrasound examination of the pelvis is considered the first-line imaging technique in the diagnosis of endometriosis [13]. In recent years, several studies have focused on PFM sonographic assessment, with techniques including 2D/3D transvaginal ultrasound (TVUS) and transperineal ultrasound (TPUS), evaluating pelvic floor morphometry in static or dynamic conditions [14], and there has been initial exploration of the use of PFM sonographic assessment in endometriosis [12,15-17]. Studies have mostly focused on evaluation and measurement of the hiatal area of the levator ani muscle [15], demonstrating PFM hypertonia and dyssynergy, which seem to be greater in patients with deep infiltrating lesions [16], or complaining of symptoms such as voiding disturbances or dyspareunia [12,17]. To date, however, no other sonographic parameter has been specifically addressed in the evaluation of PFM dysfunction related to endometriosis. Other parameters applied in PFM assessment include the anorectal angle (ARA), which depends on contraction of the puborectalis muscle “sling” [18], the morphometry of the muscle itself [19], and the features of the anal sphincter apparatus [20, 21].
The aim of our study was to describe PFM dysfunction in women with DIE assessing the puborectalis muscle (PRM) and anal sphincter muscle (ASMs) through the combined use of TVUS and TPUS in static and dynamic conditions. To this end, we compared patients suffering from DIE with healthy controls, to investigate possible differences in parameters such as the ARA, the thickness of the anal sphincters, and the PRM area.
Methods
This was a prospective case-control study conducted between June 2018 and January 2019 at the University Department of Obstetrics and Gynecology at the Mauriziano Umberto I Hospital in Turin. We included as cases 81 women with DIE affecting the USLs or the bowel, enrolled at our endometriosis outpatient clinic. Endometriosis was diagnosed according to evidence-based clinical and sonographic criteria [1,13]; in some cases, integration with other diagnostic tests, including magnetic resonance imaging, was required. The control group comprised 14 healthy women recruited during routine visits to our gynecological ultrasound outpatient clinic; in each of them, endometriosis was ruled out through clinical and sonographic examination.
The exclusion criteria were age <18 years, post-menopausal status, current pregnancy, previous vaginal deliveries, or any other condition that could potentially cause PFM dysfunction as a result of chronic pain or inflammation; these conditions included active infections of the genitourinary tract, pelvic organ prolapse, chronic inflammatory diseases or malignancies affecting the genitourinary tract and/or the bowel, pain syndromes (such as irritable bowel or interstitial cystitis), and a history of sexual abuse. In this way, we excluded possible sources of confounding effects of vaginal deliveries or conditions causing pelvic pain, which might interfere with evaluation of PFM functionality.
In the study group, anamnestic data were collected on clinical features of endometriosis, including previous surgical interventions for endometriosis and symptoms. All cases underwent a TVUS examination carried out by a single operator specialized in endometriosis (L.M.); this allowed a detailed description of location, extension and sonographic features of DIE lesions [13].
PFM ultrasound evaluation included a 2D-TVUS and a 3D-TPUS examination, performed with Affiniti 50 or Affiniti 70 ultrasound machines (Philips, Amsterdam, The Netherlands, 2013) equipped with either a C9-4v Endocavitary Probe with 4.0-9.0 MHz frequency range or a C10-3v Endocavitary Probe with 3.0-10.0 MHz frequency range for TVUS, and with a V6-2 4D Convex Ultrasound Probe for TPUS.
In all patients we obtained measures relating to PRM and ASM function. Images were acquired after complete bladder emptying and with the patient in the lithotomy position, first during static evaluation at rest, and then in dynamic conditions during Valsalva maneuver and during sustained contraction of the PFM. In order to overcome potential misunderstanding, by patients, of the movement required, we asked our patients to perform maximum Valsalva maneuver and muscle contraction in three consecutive attempts, and considered the best response in terms of greater pelvic organ displacement. The images were reviewed off-line by the same operator.
PRM function was evaluated first by measuring the ARA. The PRM forms a so-called “sling” at its insertion on the rectum, creating a measurable angle between the longitudinal axes of the rectal ampulla and the anal canal; during Valsalva the muscle relaxes allowing widening of the angle and facilitating the passage of stool, whereas in sustained contraction the angle becomes sharper, helping to maintain fecal continence [18,22]. The amplitude of the ARA was measured in degrees at rest, during Valsalva, and during sustained contraction, through TVUS imaging of the anorectal canal in the sagittal plane; the probe was inserted in the posterior fornix of the vagina with dorsal orientation on the mid-sagittal plane until visualization of the rectal wall, and then slightly withdrawn until the anorectal canal was fully imaged in the region where the ARA could be measured. Second, we recorded the presence of PRM dyssynergy, defined as an abnormal movement in response to commands given by the examiner; for a normal response the woman was required to achieve relaxation of the muscle during Valsalva, with an increase in the ARA, and a sustained PFM contraction (lasting for more than five seconds) with a reduction in the angle. Dyssynergy was deemed present when this normal pattern was not obtained; it therefore included situations in which either PRM relaxation was not achieved during Valsalva, with some cases of an opposite response resulting in a contraction of the muscle, or PFM contraction could not be maintained for an adequate amount of time. The patterns of muscular contraction were evaluated on the basis of observation of dynamic changes in the ARA after TVUS imaging of the anorectal canal as previously described. We also performed a morphometric assessment of the PRM at rest by measuring the muscle area in cm2, considering the muscular bundles in their extension up to the insertion on the ischiopubic branches. This evaluation was obtained by means of TPUS with the 3D convex probe placed translabially on a midsagittal plane, acquiring a single volume at rest and calculating the muscle area from the reconstructed image. A rendered axial plane encompassing the PRM together with the urethra, paravaginal tissues, vagina, ano-rectum, symphysis pubis and inferior pubic ramus was used for measurements, according to a previously propsed methodology for visualizing the levator ani hiatus area [12,15,19]; the muscle area was measured by highlighting the PRM bundles as a hyperechoic semi-oval image inserting ventrally on the ischiopubic branches and surrounding dorsally the posterior aspect of the ano-rectal junction [19].
ASM assessment involved measuring the thickness in mm of the internal anal sphincter (IAS) and the external anal sphincter (EAS) in static and dynamic conditions, through a TVUS introital evaluation [20]. The probe was introduced into the vagina by about 1-2 cm, rotated 90° clockwise and tilted to an angle greater than 45° in order to obtain a transverse scan of the first 3 cm of the ASM apparatus. ASM thickness was evaluated at rest and during Valsalva and sustained PFM contraction; the internal and external parts of the sphincter complex were defined according to their echogenicity as described in other works [20, 21], and measured radially at their thickest point. We did not use an endoanal probe as its introduction into the anal canal might have exerted compression on the walls of the viscera, modifying their measured thickness and the functional behavior of the muscles [21].
We compared patients suffering from DIE with healthy women, to verify whether the sonographic measurements relating to the PRM and ASM showed differences between the two groups; our hypothesis was that DIE would be associated with PFM dysfunctional changes in these parameters.
Statistical analyses
Continuous variables were expressed as mean +/- standard error; categorical variables were expressed as n (%). We compared the group of DIE cases with healthy controls, to evaluate potential differences in the features of the PFM in both static and dynamic conditions. For the comparison of continuous variables, we used a two-tailed t-test for independent samples with unequal variances, while for categorical variables a Chi-squared test was carried out; to compare more than two groups, analysis of variance (ANOVA) was performed. The choice of parametric tests was made after checking, through a Kolmogorov-Smirnov test, that the distribution of our variables did not differ significantly from the normal curve. For all tests, a difference between the groups was considered statistically significant when associated with a two-tailed p < 0.05.
Statistical analyses were performed using SPSS 22.0 software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
Ethics approval
This study was conducted in accordance with the 1964 Declaration of Helsinki. Since it did not involve any experimental research on participants, and all the procedures being performed were part of routine diagnostic care, our local ethics committee, when consulted, deemed that no formal approval was necessary.
All the patients gave their informed consent to participate in the study and for the anonymous use and publication of their data for scientific purposes.
Results
In our study, 95 women were enrolled and underwent PFM sonographic evaluation; this population included 81 cases with DIE, and 14 healthy controls with no clinical or sonographic evidence of endometriosis. The full ultrasound evaluation was carried out in all the women with good compliance. Table 1 shows the baseline characteristics of the groups in terms of anamnestic data, location of endometriosis, and reported symptoms.
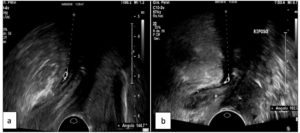
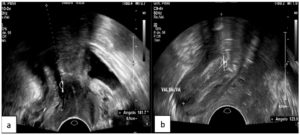
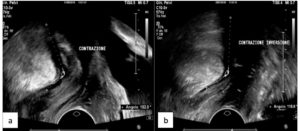
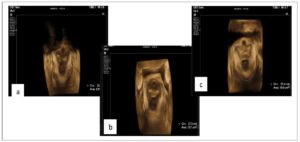
The PRM assessment results are reported in Table 2. On evaluation of the ARA, patients with DIE showed significantly lower values both at rest (p 0.02) and during Valsalva (p <0.001), and reduced widening of the angle during this maneuver (p <0.001); Figures 1 and 2 show examples of these observations. In the sustained contraction condition, the ARA showed a minor variation from baseline (p 0.015). PRM dyssynergy was reported only among women with DIE, specifically in 81.5% of the cases. The patterns observed included (1) coactivation of levator ani complex muscles during Valsalva, including the PRM, resulting in a reduction rather than a widening of the ARA; (2) failure to maintain sustained PFM tone, with discontinuous contractions alternated with intermittent relaxation, or complete immobility of the muscle; (3) reverse response to command, resulting in PFM contraction in response to a request to perform a Valsalva maneuver, and vice versa (Fig. 3); (4) use of accessory muscles during contraction (i.e. gluteal or adductor muscles). On reconstruction by 3D-TPUS, the mean area of the PRM bundles was significantly higher (p <0.001) in patients with DIE (Fig. 4).
Table 3 shows the ASM assessment results. During dynamic evaluation, healthy subjects showed thickness changes in both ASMs, consisting of thickening of the sphincters when they were relaxed during Valsalva, and thinning during sustained contraction; the same dynamic pattern was reported in patients with DIE. For both the IAS and the EAS, differences in thickness between the resting, Valsalva, and contraction conditions were statistically significant on the ANOVA test for repeated measures (p <0.001), both in cases (F 8.54 for IAS, F 7.96 for EAS) and in controls (F 16.22 for IAS, F 35.10 for EAS). Comparison between the two groups did not show significant differences in the thickness of the IAS or the EAS at rest. Overall, the dynamic response of the sphincter apparatus appeared to be significantly better in healthy women, who showed greater sphincter thickness during Valsalva for both the IAS (p 0.019) and the EAS (p 0.007), and a thinner EAS during contraction (p <0.001), when compared with patients with DIE.
Discussion
In our study we have demonstrated that patients with DIE affecting the USLs or bowel, when compared with healthy women, show sonographic signs of PRM and ASM dysfunction.
Our evaluation of the ARA, which directly depends on the tone of the puborectalis sling [18], suggests the presence of PRM hypertonia in DIE. This was highlighted at rest, when the angle was found to be sharper than in healthy controls, and during Valsalva maneuver, when the PRM failed to properly relax and widen the angle, leading to an alteration in the width of the anorectal canal. At the same time, contractile capacity was found to be reduced in the DIE group, probably because, in this condition, the PRM is already in a state of tonic contraction and loses the ability to contract further. These data are consistent with the findings of previous studies investigating a sonographic parameter reflecting the same mechanism, namely the levator ani hiatal area (LHA) [12, 15-17]. The LHA is in fact delimited by the PRM and pubic ramus, and varies dynamically according to the tone of the PRM. In women with DIE, increased PFM tone at rest, failure of relaxation during Valsalva, and decreased PFM contractile strength have been demonstrated through evaluation of the LHA [15]; our ARA assessment data show the same pattern. To evaluate the LHA, it is necessary to acquire a 3D volume and perform image reconstruction, which means that a convex volumetric transducer is required for TPUS. Compared with the LHA, the ARA might be a simpler and more readily available parameter, as it can be assessed through 2D-TVUS examination.
Increased PRM tone might be included in a wide range of PFM dysfunctional disorders encompassing hypertonicity, failure to relax and tenderness [6, 23]. These disturbances usually depend on continuous inflammatory and nociceptive stimulation – as in women with DIE – causing antalgic contraction, with continuous recruitment and tonic activation of muscle groups [23]. In DIE, the nociceptive stimulation does not depend only on the features of the lesion itself, such as its extension and infiltrating behavior; pain generation is also due to inflammatory and neuropathic processes leading to sensitization, suggesting a remodeling of neural pathways [24]. These mechanisms include increases in nerve fiber density and activity in the environment surrounding endometriotic lesions, as a result of cytokines and growth factors produced in chronic inflammation; at the same time, sensitized nociceptive neurons may help to maintain inflammation through the release of neuropeptides [24, 25]. Through these phenomena, persistent neuropathic pain circuits are established, causing a self-maintaining painful stimulus, and leading to a constant muscle contraction [23, 25]. This can contribute to impairment in sexual, urinary and defecatory functions [6, 12, 16, 23].
Our dynamic evaluations of the PRM frequently highlighted the presence of abnormalities during the performance of motor tasks. The most common problem was a coactivation of the abdominal and pelvic floor muscles during Valsalva; this has been previously described as a paradoxical contraction of the PRM, which can obstruct the normal passage of stool [26], and appears to be more frequent in women with endometriosis [15]. During the task of sustained contraction, several women showed intermittent relaxation of the PFM, and were therefore unable to continuously maintain adequate tone, or even absence of contraction, resulting therefore in impairment of PFM endurance [27]. In some cases, we also observed reverse responses to command, with a further increase of PRM tone during Valsalva, and relaxation of the muscle when contraction was requested, or recruitment of accessory muscles to perform the task. These phenomena could reflect impairment of the capacity for muscular isolation, that is the ability to contract specific muscles individually in order to carry out a command [27]. Patients with DIE have been shown to present reduced PFM contractile strength [15,16], which our study confirmed. Furthermore, the neuroinflammatory damage might also affect the ability to correctly recruit different muscle groups, resulting, over time, in impairment of the synergistic activation of muscles necessary to achieve a motor task [27]. Paradoxical contraction and dyssynergy of the PFM are known mechanisms underlying disorders of defecation [22]. It is noteworthy that we observed PFM dyssynergy in 81.5% of cases, although only 55.6% reported defecatory symptoms; this suggests frequent PFM anomalies in women with DIE, who might often be unaware of their dysfunctional muscle behavior.
Morphometry of the PRM showed a greater muscle area in the DIE patients. It can be hypothesized that this greater surface could be an expression of muscular hypertrophy and hyperplasia induced by tonic contraction, or even by inflammatory cytokines and growth factors. A recent study assessing PFM through transperineal elastography showed that patients with DIE had decreased elasticity of these muscles, which was linked to persistent spasm [28]; it cannot be excluded, however, that continuous neuroinflammatory stimulation might lead to actual structural changes in PFMs, resulting in stiffer and dysfunctional muscles.
ASM abnormalities observed in our study population include a reduced capacity for dynamic changes in thickness during Valsalva and contraction. Few studies have performed dynamic evaluation of anal sphincters using ultrasonography; the available evidence shows a close relationship between ASM changes and PRM activity [29]. In patients with endometriosis, no study has yet evaluated ASM function through sonographic parameters. Studies investigating the findings of anorectal manometry applied in DIE showed hypertonia of the IAS and reduced squeeze pressure exerted by the ASM, which likely derived from difficulty further contracting muscle that is already in a state of persistent activation [9, 30]; this mechanism may actually mirror our findings on the PRM.
Our study had some limitations. First, the population was limited in size, especially the healthy controls; moreover, among the cases, the diagnosis of pelvic endometriosis was based mainly on clinical and sonographic criteria, since the majority of the patients did not undergo surgical exploration to obtain histological confirmation of the disease. Second, some of the parameters evaluated, such as dynamic changes in the ASM or the type of dysfunctional movements of the PRM, have not yet been extensively studied in the general population, making it difficult to draw conclusions about patterns observed in DIE cases. Finally, since all the examinations were performed by a single expert operator, we were unable to provide data regarding the reproducibility and interobserver variability of this type of sonographic assessment.
Despite these limitations, our study highlights sonographic findings and dysfunctional mechanisms, which could be implicated in the pathogenesis of endometriosis-associated symptoms. These mechanisms seem to suggest a PFM dysfunction encompassing muscular hypertonia at rest and failure to properly relax; reduced contractile capacity; and dyssynergic contraction of muscle groups. The majority of these parameters can be evaluated through TVUS without requiring 3D/4D probes, which could make sonographic evaluation of PFM available in a wider range of contexts, i.e., as a first-level test to identify patients in whom it would be worth performing a more in-depth diagnostic evaluation. The application of this evaluation to women with DIE, besides allowing mapping of endometriosis lesions, could help to define mechanisms underlying pain and functional impairment symptoms, especially in those cases with persistence of the disorders even after surgical or medical treatments. This could help in directing women towards individualized and targeted functional rehabilitation pathways. Further studies are needed to confirm these results on a larger population, investigating the variability of sonographic evaluation between operators, and identifying differences with regard to DIE location or reported symptoms.
Acknowledgments
No specific funding was obtained for this study.
Conflict of interest
The authors declare that there is no conflict of interest.