Introduction
With the tendency toward younger menarche, lower birth rates, and longer lives, contemporary women spend far more of their lives menstruating than their ancestors did. According to Mishell [1], women have approximately 481 menstrual cycles during their reproductive years. With an average adjustment of 22 months for two pregnancies and postpartum periods, many women actually experience about 459 cycles during their childbearing years. While regular menstruation could serve as an index for overall reproductive health, every woman who ovulates knows, to some extent, the menstrual cycle’s influence on the physiological, psychological, and/or social aspects of her life.
The present authors conducted a series of investigations to explore psycho-neuro-endocrinological fluctuations during the menstrual cycle [2-5]. The authors’ retrospective [6] and prospective [7] epidemiological surveys demonstrated that 99.5% and 85.5% of participating healthy college students experienced at least one symptom in the late-luteal phase, respectively. A constellation of diverse symptoms appearing in the late-luteal phase and known as premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD) can impact an individual’s interpersonal relationships, social interactions, occupational and/or domestic activities, and diminish the affected woman’s quality of life [8-10]. Even when the severity of the symptomatology does not reach the diagnostic criteria for PMS or PMDD, some more severe symptoms women experience premenstrually are strongly associated with negative subjective perception of health and stress [6]. Such premenstrual symptoms, especially in young women, can be associated with academic performance impairments, including absenteeism [11] and poor grades [12,13]. Further, the constellation of symptoms renders affected women more vulnerable to negative health events in later years, such as postpartum depression [14] and menopausal symptoms [15]. In addition to the specific menstrual phase, such as the late-luteal one, what other factors influence women’s mind and body health? Identifying potential detrimental factors could contribute to enhanced health education for young women, and ultimately, to better overall wellness throughout a woman’s life.
Retrospective assessment has been widely used in cross-sectional epidemiological women’s health research [6,11-13,16-19]. This tool effectively gathers various kinds of information; however, we cannot deny that a participant’s bias may affect her questionnaire responses [7]. Therefore, the authors designed a prospective observational study to scrutinize potential factors associated with menstrual-cyclic symptomatology among college students. A previous retrospective survey by the authors clarified that a less-than-healthy lifestyle, including, for example, skipping breakfast, could undermine biopsychosocial health, especially in the premenstrual phase in women of early reproductive age [6]. This finding encouraged the authors to further explore, in the current study, how and to what extent sleeping habits, another representative factor influencing mind and body health [20], affect the symptomatology during the menstrual cycle.
Methods
Study design and participants
One hundred nineteen college students, who had responded to a campus advertisement, enrolled in a series of women’s health research projects, including 2019 and 2021 epidemiological surveys [6,7]. All subjects received an explanation of the nature and purpose of the present prospective observational investigation, and 69 subjects agreed to participate in the study. The Institutional Review Board of Shitennoji University approved the present study protocol. All study procedures complied with the ethical standards of the Helsinki Declaration of the World Medical Association. Before receiving any data about the experiments, all subjects provided written informed consent to participate in the study.
The subjects first underwent a brief face-to-face interview and completed a standardized health questionnaire regarding medical history, medication, current health condition, regularity of menstrual cycles, and lifestyle.
None of the college students had been clinically diagnosed with gynecological problems, such as amenorrhea, dysmenorrhea, and endometriosis. No subjects reported taking oral contraceptives to control their menstrual cycles. None of the women had been clinically diagnosed with diabetes mellitus, hypertension, hyperlipidemia, or other lifestyle-related diseases. None suffered from any psychiatric diseases.
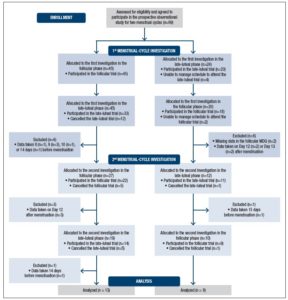
The subjects came to the laboratory four times in total to participate in the observational prospective study over two menstrual cycles. As Figure 1 shows, subjects were examined on two separate occasions in each investigation: once during the follicular phase (the fifth to the eleventh day from the first day of menstruation) and once during the late-luteal phase (within seven days before the next menstruation), in accordance with the authors’ previous studies [7,21-24].
On the basis of the subjects’ self-reported regular menstrual cycles, we determined the cycle phase during the experiments by the onset of menstruation, together with oral temperature (MC-172L, Omron, Kyoto, Japan), and concentrations of ovarian hormones, estrone conjugates, and pregnanediol-3-glucuronide (PdG) in a urine sample, taken early in the morning. Both estrone and PdG were indexed to creatinine (Cr) excretion [7,21-24]. Considering subjects’ menstrual cycles and availability to participate in this research project, the order of testing was randomized: 45 subjects were allocated to the investigation in the follicular phase followed by the late-luteal phase. The remainder of the subjects were tested in reverse order (Fig. 1).
On the days of the follicular and late-luteal trials, subjects came to the laboratory between 8:00 and 11:00 after having fasted overnight. Height and body weight of each subject were measured to calculate body mass index (BMI) as body weight divided by height squared. The subjects then filled out the Menstrual Distress Questionnaire (MDQ) to evaluate the prevalence and severity of biopsychosocial symptoms on the day of the experiment [25]. Briefly, the MDQ explores 46 symptoms on a six-point scale ranging from no experience of the symptom to experiencing it at its most severe level. The total scores could, therefore, range from a minimum of 46 points to a maximum of 276 points. The authors also asked the subjects to report their bedtime the night before the trial and their wakeup time on the day of the trial to investigate the effect of sleep duration and/or sleep time on the symptom complex on the day of the experiment.
Statistical analysis
All descriptive and inferential statistical analyses were performed using a commercial software package (IBM SPSS Statistics Version 25; IBM Corp., Armonk, NY, USA). Internal consistency of the MDQ was evaluated by calculating Chronbach’s alpha coefficients. Multiple regression analysis was performed to examine factors potentially related to biopsychosocial symptoms assessed by the MDQ. The effects of ‘cycle’ and ‘menstrual phase,’ and their interaction, were evaluated using two-way analysis of variance (ANOVA) to investigate the influence of these two factors on the subjects’ clinical characteristics. Paired t-test was performed to compare urinary ovarian hormone concentration in the follicular and late-luteal phases. Values are reported as means ± standard deviations. Statistical tests were two-sided, and p < 0.05 was adopted as the level of significance.
Results
Figure 1 shows the flow of participants from recruitment through completion of the present study. Although 69 subjects agreed to participate in the prospective observational study for two menstrual cycles, 18 and 12 participants, in total, canceled the first and second investigation, respectively. The authors excluded a further 17 subjects for several reasons, such as missing data on the MDQ and inability to present in the follicular or late-luteal phase. This study, therefore, analyzed data taken from 22 participants (mean age: 20.5 ± 1.1 years), who completed the four trials: two trials in the first followed by two trials in the second menstrual-cycle investigation.
Table 1 shows the clinical characteristics of the subjects in the follicular and the late-luteal phases. Body weight, BMI, and basal body temperature significantly increased from the follicular to the late-luteal phase. Statistical analysis with ANOVA revealed no significant effect of cycle or interaction effect (cycle x menstrual phase) on these clinical characteristics. The authors also measured urinary ovarian hormone concentrations during the menstrual cycle. Estrone conjugates (ng/ml Cr) significantly increased in the late-luteal (19.75 ± 15.96) from the follicular phase (10.94 ± 4.49) (mean difference -8.81, 95% confidence interval -15.92 to -1.70, p = 0.018). The values of PdG (μg/ml Cr) in the late luteal phase (2.59 ± 1.69) were also significantly greater than that in the follicular phase (0.53 ± 0.25) (mean difference -2.06, 95% confidence interval -2.79 to -1.33, p < 0.001).
As mentioned in the Methods, all participants had no significant health problems and self-reported that their menstrual-cycle fluctuations were within the range for regular menstrual cycles (25 to 38 days), as defined by the Japan Society of Obstetrics and Gynecology [26]. During the two menstrual-cycle prospective investigations, however, the authors observed eight cases that slightly deviated from a regular menstrual cycle; seven with shorter cycles (22 to 24 days) and one with a longer cycle (39 to 40 days) (Table 2). Considering the first and second investigations, the subjects displayed the following menstrual regularity patterns: shorter and shorter (n = 3), shorter and regular (n = 1), regular and regular (n = 17), and longer and regular (n = 1).
The Cronbach’s alpha coefficient values for the MDQ in the first follicular, first late-luteal, second follicular, and second late-luteal trials were 0.90, 0.92, 0.91, and 0.93, respectively.
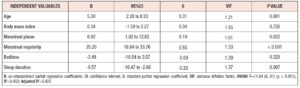
Referring to a 2019 epidemiological survey [6], we performed multiple linear regression analysis to determine how well the combination of six independent variables (age, BMI, menstrual phase, menstrual regularity, sleep schedule, and sleep duration) explained the variance in the MDQ total scores. As regards sleeping habits, the subjects showed the following average and the median (quartile range) sleep duration values: 5.92 ± 1.28 and 6.00 (2.00) hours, respectively. Thus, the authors dichotomized the variable, shorter (0) or longer (1) than six hours, for the statistical procedure. In addition, sleep schedule ranged from 22 pm to 4 am among the subjects. Since the median (quartile range) of the sleep schedule was 1.00 (2.00) am, “before 1 am” and “after 1 am” were assigned to ‘0’ and ‘1,’ respectively. The other two variables were dichotomized as follows: menstrual phase (follicular = 0, late-luteal = 1) and menstrual regularity [regular = 0, irregular (shorter or longer) = 1]. As Table 3 shows, multivariable analysis revealed age (β = 0.31, p = 0.001), menstrual phase (β = 0.19, p = 0.022), menstrual regularity (β = 0.55 p < 0.001), and sleep duration (β = -0.26, p = 0.007) as the factors significantly related to the severity of biopsychosocial symptomatology evaluated by the MDQ among college students.
Discussion
The present prospective observational investigation found significant factors, including menstrual phase and menstrual regularity, sleep duration, and age, affecting biopsychosocial symptoms among the college students investigated. A number of epidemiological surveys conducted worldwide have explored mind and body health and related cultural and environmental factors among women of early reproductive age. As for menstrual-cyclic symptomatology, the outcomes have been reasonably congruent, although research designs (retrospective or prospective) and methods differ among studies. Investigations from 2013 to 2021 indicate that regardless of ethnicity, more than 85% of women in their teens and twenties experienced at least one cyclical symptom in the late-luteal phase, generally known as premenstrual symptom or PMS [6,7,16-19,27]. As the current authors’ findings together with the above substantial evidence have clarified, during the late-luteal phase, biopsychosocial and behavioral conditions could be impaired in reproductive-age women, even those without major medical disorders. For research purposes, in addition, this result further indicates that the influence of menstrual cycle phase should be kept in mind when data are gathered, and, ideally, the menstrual phase should be documented in detail [28].
Irregular menstrual cycles are defined as abnormal variability or variation in length [29]. Prior to the prospective investigations, we conducted face-to-face interviews and paper-based questionnaire surveys and confirmed that all the women in the present study menstruated regularly. During the observations of two menstrual cycles, however, the cycles of five women deviated slightly from the Japanese criteria for a regular menstrual cycle [26]. Menstrual cyclicity depends on stable release of hormones by the hypothalamic-pituitary-gonadal (HPG) axis. Psychobiological research has revealed that traumatic or enduring stressful events and a variety of chronic psychosocial stressors affect the HPG axis and are associated with disrupted menstrual functioning, including menstrual cycle irregularity [29-32]. In the present study, the authors conducted a follow-up comparison of clinical features, including basal body temperature and urinary PdG, between women with regular (n = 17) and irregular menstrual cycles (n = 5). The statistical analysis with two-way ANOVA revealed no significant between-subjects factor in these variables (basal body temperature: F = 2.77, p = 0.10; urinary PdG: F = 3.51, p = 0.08) although the late-luteal increase was lower in women with menstrual irregularity [basal body temperature (℃): regular Δ 0.35 ± 0.25 vs. irregular Δ 0.23 ± 0.38; urinary PdG (μg/ml Cr): regular Δ 2.42 ± 1.70 vs. irregular Δ 0.84 ± 0.52]. On the basis of these findings, the authors cannot demonstrate that the women with irregular menstrual cycles in the present study suffer from hypothalamic/pituitary or ovary/uterine failure. Indeed, more detailed scrutiny of a greater number of participants would be needed in order to study women’s psycho-neuro-endocrinological features during the menstrual cycle along with their clinical backgrounds. Although the authors acknowledge the shortcomings of the present investigation, the results of the present study combined with previous psychobiological stress research [29-32], mentioned above, imply that even a slight deviation of menstrual regularity could be a precursor to altered health conditions.
Good sleep, a critical lifestyle habit, is essential to mind and body health [20]. The present study indicates that less than six hours of sleep at night could acutely and negatively impact biopsychosocial symptoms the next morning. Although sleep requirements vary among individuals, it is recommended that adults aged 18 to 60 years sleep at least seven hours each night to promote optimal health and well-being [33]. Sleeping less than seven hours per night is associated with adverse health outcomes, including weight gain and obesity, diabetes, high blood pressure, coronary heart disease and stroke, and increased risk of death [33]. Lack of sleep not only increases fatigue and sleepiness among students, it also worsens cognitive performance, including memory consolidation, grades, and study effort [34]. Inadequate sleep can also cause frequent mental distress and behavioral problems [33]. A 2018 population-based epidemiological survey additionally revealed that reproductive-age women with sleep duration of less than five hours a day, in addition to psychological stress, depressive mood, or suicidal ideation, have a higher probability of menstrual-cycle irregularity [32]. Good sleep health is characterized by adequate duration together with subjective satisfaction, appropriate timing, high efficiency, and sustained alertness during waking hours [20]. Sleep inconsistency (sometimes called “social jet-lag”), defined by inconsistency in sleep schedule and/or duration from day to day, is another index used to estimate sleep health in women since greater sleep inconsistency is associated with more severe menstrual symptoms [35]. A multifaceted approach is necessary to explore an association between menstrual-cyclic sleep patterns and ovarian-hormonal fluctuations; however, the present study has reconfirmed previous sleep research outcomes [20,32-34] and underlines the importance of an adequate amount of sleep — at least six hours per night, regardless of menstrual cycle — for maintaining optimal health conditions in women.
The following limitations of the present study deserve mention. First, the present study applied a prospective approach to observe menstrual-cyclic symptoms among college students. All participants completed four trials; however, the study employed a relatively small sample, which could limit the generalizability of the study outcome. To increase the accuracy and reliability of the present research, the authors need to conduct a future study in a larger sample of women. Furthermore, we found ‘age’ to be a significant factor associated with biopsychosocial symptoms during the menstrual cycle among college students with a small age range (from 19 to 22 years). The current research design unfortunately does not allow us to interpret this intriguing phenomenon — i.e., to clarify why age affected menstrual-cyclic symptoms in participants falling within this short three-year age span. As well as increasing the number of subjects, ideally, a longitudinal cohort study should be conducted to assess women’s health from menarche to menopause, while observing hormonal fluctuations together with biopsychosocial symptoms and other environmental factors.
Second, in each of the two menstrual cycles considered, we examined the subjects on two separate occasions: once during the follicular phase and once during the late-luteal phase. Severity of symptoms and lifestyle-related behaviors can fluctuate even within menstrual phases. Thus, we should improve the research design to collect more data during the menstrual cycle. Taking the recent availability of web-based mobile apparatuses into consideration [36], the present study further suggests the need to develop an accurate, convenient, and, if possible, user-friendly, real-time women’s health self-monitoring system.
Finally, as previous research elucidated [28], menstrual cycles have various effects on sleep. The present study evaluated sleep schedules and duration, however, the quality of sleep, including subjective satisfaction and sleep continuity, is also critical to general health. Sleep conditions should thus be evaluated using subjective scales and also objective measurements, such as polysomnography. In addition, to promote women’s mind and body health further, it would be of interest to probe multidimensionally an interrelationship among circadian rhythm, sleep habits, and sexual-reproductive health.
Conclusions
The findings of the present study indicate that the late-luteal phase is a significant factor in biopsychosocial symptoms among reproductive-age women without major health problems, including severe PMS or PMDD. A slight deviation from the regular menstrual cycle might potentially be a sign of mental and physical health alterations. This study reconfirms the importance of adequate sleep duration for maintaining optimal health and well-being in female college students. Taken together, the current research further suggests the value of future implementation of educational programs on menstruation and its effects on women, and on healthy lifestyle habits and behaviors aimed at improving fitness and wellness, and ultimately, the quality of women’s lifelong health.
Acknowledgments
The authors express their appreciation to all the volunteers for their dedicated participation in this study. The authors also thank Ms. Hitomi Nakata, a research assistant, for organizing and reviewing data obtained in the current study. This work was funded by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C) 18K11086.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.