Introduction
In pregnancy, the fetus can be regarded as an allogenic graft that requires careful adjustment of the maternal immune system [1]. Temporary immune suppression is a normal mechanism during fetal development and tissue repair. The immune suppression that occurs during pregnancy is a dynamic modulation involving feto-maternal immunological cross-talk that protects the fetus from the maternal immune system [2]. Recent studies have identified a balanced interaction between pregnancy hormones, microbiota, and the maternal immune system that is essential for homeostasis, tolerance of a semi-allogenic fetus, and successful delivery [1,3].
The act of initiating fertilization, thus establishing a pregnancy, involves a cascade of leukocyte cytokine-mediated events that cause a transient shift towards a more permissible maternal immune system [4]. This cascade inhibits potentially harmful cell-mediated immune activity against paternal antigens associated with conception, which in part, may play a role in facilitating implantation and development of the placenta [5]. The normal progression of a successful pregnancy is partitioned into three sequential steps: pro-inflammatory, anti-inflammatory, second anti-inflammatory [6]. The pro-inflammatory response is mainly characterized by Th1 cytokine patterns, with IFN-γ, IL-2, IL-3, TNF-α, and TNF-β as products while the anti-inflammatory response is characterized by Th2 cytokine patterns, with secretion of IL-4, IL-10, IL-5, IL-9, and IL-13. Various complications, including preterm birth, pre-eclampsia, and miscarriage, can stem from the maternal immune system not responding to specific pregnancy stages, to pneumonia, and to viral or bacterial infections [6].
An overall decrease in pro-inflammatory cytokines and increase in counter-regulatory cytokines is known to occur during uncomplicated pregnancies [7]. Although the careful modulation of the immune system during pregnancy has been documented, there is no clear evidence to state whether these immunological changes remain, or whether there is a postpartum return to prior cytokine expression. Medawar first identified the importance and specificity of immune responses during pregnancy in 1953 [8]. While most of the literature focuses on studying the vaginal immune system during pregnancy and the potential pathology associated with disturbed immunity, not much is known regarding particular immunological differences between women who have never had a pregnancy and women who have had a successful pregnancy or pregnancies.
The purpose of this research is to identify whether there is a measurable difference in cytokine expression in women who have had a successful pregnancy or pregnancies and are not currently pregnant versus women who have never become pregnant. Associations between parity and immunology have the potential to offer insight toward understanding the complex immune system within the vagina, how it responds to pregnancy, and if it changes postpartum.
Materials and methods
Human subjects and data collection
Vaginal samples were collected to study vaginal conditions. The samples were obtained from the middle of the vagina using standardized cotton swabs [9,10]. Vaginal specimens were placed in 1 ml of physiological solution (phosphate-buffered saline) and stored at -80 °C. Vaginal samples were collected from eleven women in the parous group and from seven women in the nulliparous group. The study was IRB (Institutional Review Board) approved (IRB protocol# L20-225) at Texas Tech University Health Sciences Center, TX, USA.
Cytokine evaluation
Cytokine analysis was carried out using two instruments: 1) the Bio-Plex MAGPIX multiplex reader instrument (Bio-Rad, USA); 2) the MesoQuick Plex SQ 120 instrument (Meso Scale Discovery, MD, USA). The authors have previously published a detailed description of the relevant methods [11].
1) The Bio-Plex Pro Human cytokine 27-Plex Immunoassay is a 96-well kit (Cat# M500KCAF0Y, Bio-Rad, USA) that includes magnetic beads, detection antibodies, wash buffer, sample diluent, detection antibody diluent, a flat bottom 96-well plate, and plastic adhesive plate sealing tape [11,12]. The plate was pre-wetted with 100 µl of Bio-Plex assay buffer. 50 µl of working bead solution was added into each well. The plate was washed with 100 µl of Bio-Plex wash buffer (2X). Then, 50 µl of standards and 50 µl of samples were added to the appropriate wells of the plate. The plate was covered with plastic adhesive plate sealing tape and incubated for 30 min at room temperature with shaking. The plate was then washed with 100 µl of Bio-Plex wash buffer (3X). 25 µl of Bio-Plex detection antibody diluent was added to each well of the plate. The plate was again covered with plastic adhesive plate sealing tape and incubated for 30 min at room temperature with shaking. Next, the plate was washed with 100 µl of Bio-Plex wash buffer (3X). 50 µl of Streptavidin-PE working dilution (100X) was added to each well of the plate. The plate was covered with plastic adhesive plate sealing tape and incubated for 10 min at room temperature with shaking. Again, the plate was washed with 100 µl of Bio-Plex wash buffer (3X). The beads were re-suspended in each well with 125 µl of Bio-Plex assay buffer. The plate was covered with plastic adhesive plate sealing tape and shaken at 1100 rpm. Finally, the tape was removed and the plate was immediately read using the Bio-Plex MAGPIX multiplex reader instrument (USA).
2) MSD (Meso Scale Discovery) cytokine assays provide a rapid and convenient method for measuring cytokine levels within a single, small-volume sample [11,12]. An MSD 96-well plate was pre-coated with capture antibodies on independent and well-defined spots. All vaginal swab samples were analyzed using the MSD multiplex instrument MESO QuickPlex SQ 120 (MSD, MD, USA) [13]. The MSD electrochemiluminescence (ECL) detection system has been validated for cytokine measurement in vaginal swab samples [12].
A total of 5 custom plates were prepared for the multiplex assays:
1) Plate 1: IFN-γ (interferon-γ), IL-1β (interleukin-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL- 12p70, IL-13, TNF-α (tumor necrosis factor-α)
2) Plate 2: GM-CSF (granulocyte-macrophage colony-stimulating factor), IL-5, IL-7, IL-15, IL-17A
3) Plate 3: Eotaxin, MIP-1α (macrophage inflammatory protein-1α), MIP-1β, MCP-1 (monocyte chemoattractant protein-1)
4) Plate 4: VEGF-A (vascular endothelial growth factor-A), bFGF (basic fibroblast growth factor)
5) Plate 5: IL-1RA (interleukin-1 receptor antagonist), IL-9
The whole kit (Cat # N05049A-1, MSD, USA) was provided by MSD to perform multiplex assays and to detect a total of 23 cytokines in all samples. The plate was washed 3 times with 150 µl/well of wash buffer. 50 µl of samples, calibrators, or controls was added into each well of the plate. The plate was sealed with adhesive plate sealing tape and incubated at room temperature for 2 h on a shaker at 700 rpm. The plate was then washed 3 times with 150 µl/well of wash buffer. 25 µl of detection antibody solution was added to each well, and the plate was sealed and incubated at room temperature for 2 h on a shaker at 700 rpm. The plate was then washed 3 times with 150 µl/well of wash buffer. 150 µl of read buffer was added to each well of the plate. Finally, the plate was analyzed using the MSD multiplex instrument.
Statistical analysis
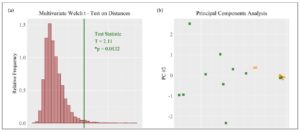
Statistical analysis was performed using RStudio with a significance level of α = 0.05 and two-sided p-values. The research hypothesis was that cytokine concentrations differ between parous and nulliparous groups. Individual differences in cytokine concentrations were measured using the permutational unequal variance t-test and standardized difference of means (SDM). The multivariate difference between the groups was visualized with a principal component analysis (PCA) plot. Univariate differences were visualized by means of side-by-side bee swarm plots. Cytokine concentrations were standardized prior to multivariate analysis. The distributions of cytokine concentrations included distributions characterized by zero-inflation, right skewness, non-normality, outliers, unequal variances, and expression on different scales [11]. The multivariate Welch’s t-test based on Euclidean distances is suitable for working with zero-inflated, right skewed, high-dimensional, and unbalanced sample data with the possibility that within-group covariance patterns are heterogeneous [14]. This allows for a single multivariate p-value testing the null hypothesis of equal group centroids for cytokine concentrations. Computations were completed using the R code provided by Hamidi et al. [15]. The multivariate Welch’s t-test was visualized with a histogram of 10,000 null hypothesis permutation statistics and the observed test statistic.
Results
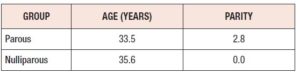
Subjects’ demographics: All patients were seen at Texas Tech Physicians Clinic, Odessa, TX and Midland, TX, and formed a primarily Hispanic population. There was no significant difference in age between the two groups. The average parity of the parous group was 2.8 (Table 1).
Cytokine concentrations differed between the groups: Eleven women were included in the parous group, whereas only seven women were included in the nulliparous group. The multivariate Welch t-test using Euclidean distances resulted in acceptance of the research hypothesis (*p=0.0132) with a PCA plot showing clustering based on patient group (Fig.1). It showed that there was a clear difference in cytokine profiles between the two groups.
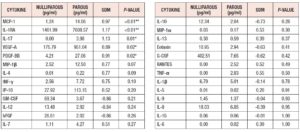
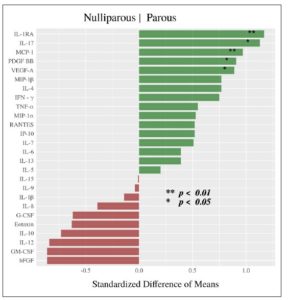
Cytokine expression was higher in the parous group: Table 2 and Figure 2 summarize the difference in cytokine profiles found between the groups. Cytokines MCP-1 (SDM = +0.968, **p=0.007), IL-1RA (SDM = +1.171, **p=0.010), IL-17 (SDM= +1.126, *p = 0.013), VEGF-A (SDM= +0.887, *p=0.021), and PDGF-BB (SDM= +0.909, *p=0.022) were significantly higher in the parous group (Fig.3).
Discussion
This study measured cytokine profiles in vaginal swab samples taken from parous and nulliparous women. During a normal pregnancy, the maternal immune system adjusts to prepare for successful gestation and delivery. In general, cytokines play an essential role in permitting the implantation of the embryo after fertilization [16]. In our study, five cytokines were observed to be significantly elevated in the parous group: IL-17, IL-1RA, MCP-1, VEGF-A, and PDGF-BB. IL-17 is a major pro-inflammatory cytokine in the human placenta that plays a critical role in angiogenesis and immune regulation. Previous studies have demonstrated a novel role of IL-17 in controlling the maternal-fetal relationship, and sustaining a normal pregnancy by inducing the production of matrix metalloproteinase and stimulating placenta formation [17]. Nevertheless, studies also show that excessive expression of IL-17 in pregnancy can lead to complications such as fetal growth restriction and preeclampsia [18].
Furthermore, the results of our study showed that IL-1RA, a natural inhibitor of the pro-inflammatory effect of IL-1β, was significantly inclined increased in the parous group compared with the nulliparous group. IL-1RA is present in amniotic fluid and cord blood and circulates systematically at higher levels in pregnant women [19]. In late pregnancy, IL-1RA has been found to significantly decrease spontaneous labor by inhibiting IL-1β-induced prostaglandin E-2 (PGE-2) production [19,20]. Interestingly, Lei et al. also noted that during pregnancy treatment with exogenous IL-1RA can protect against placental and neurodevelopmental defects induced by in utero inflammation [21].
Moreover, we observed that the expression of cytokine MCP-1, also known as chemokine ligand-2 (CCL2), was significantly higher in the parous group. MCP-1 is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages. It plays a crucial role in tumor angiogenesis and maintaining a normal pregnancy from implantation to parturition. In the first trimester, MCP-1 is secreted by trophoblast cells that are found in the decidua, chorion, and amniotic fluid [22,23]. Cytotrophoblasts and extravillous trophoblast cells increase MCP-1 secretion between weeks 8 and 10 of pregnancy and during weeks 12-14 [22,24]. Improper thrombin regulation and unregulated MCP-1 secretion during pregnancy might cause inadequate vascular formation and transformation of spiral arteries, which may contribute to preeclampsia [22].
In addition, the results of our study showed VEGF-A and PDGF-BB expression to be significantly elevated in the parous group compared with the nulliparous group. VEGF-A acts on endothelial cells inducing angiogenesis, increased vascular permeability, vasculogenesis, vascular remodeling, and cell growth; it also promotes cell migration and inhibits apoptosis. Expression of VEGF-A, along with other VEGF cytokines, declines drastically in the maternal circulation during pregnancy; this is followed by a post-partum rise. It has been found that the vast majority of VEGF is bound non-reversibly in the amniotic fluid during pregnancy [25]. The relative overexpression of post-partum VEGF-A may be the result of an abundant amount of VEGF being released into the bloodstream once it can no longer come into contact with the binding factors. PDGF-BB regulates cell growth and division and contributes to blood vessel formation and mitogenesis, while also playing a vital role in differentiating and guiding directed and non-directed migration at the implantation site [26]. VEGF-A and PDGF-BB both play a vital role in pregnancy by mediating the formation of the necessary vascular tissue at the fetal-maternal interface.
The elevated markers identified here could be related to the documented correlations between parity, fecundity, and fecundability. On the other hand, uncontrolled expression of proinflammatory cytokines can contribute to pathogenesis: e.g., unregulated elevation of Th17 responses during pregnancy has been correlated with complicated pre-term labor as well as rejection of implantation of a fertilized embryo and other autoimmune pathologies [27].
These elevated cytokine levels could be merely correlative with parity. Cytokine elevations could be caused by confounding variables not addressed in this study. Participant age, lifestyle behavior and timeline of pregnancies were not controlled in this study. It is also possible that the measured cytokine levels reflect a dynamic cytokine expression trend approaching a shift to a new baseline, i.e., a baseline different from before, when the parous participants were nulliparous. Changes in individual cytokine concentrations across gestation and life course are not well described. This research also does not investigate the cause or timing of increased cytokine expression or whether the elevations are for betterment. However, it does determine that there is a step change in cytokine expression when comparing the nulliparous and parous groups. Whether the levels of these five cytokines are deliberately increased by the postnatal immune system, or whether this is simply an after effect of pregnancy cannot be determined in this paper. More research is required to clarify the functionality of measuring cytokine levels as an indicator of pregnancy health.
In conclusion, we compared the cytokine concentrations of parous and nulliparous women. We found significant increases in IL-17, IL-1RA, MCP-1, PGDF-BB, and VEGF-A concentrations in parous women when compared with nulliparous women. There is very little in the literature that may explain what role the increased cytokines play in post-partum women or whether these have any influence on fertility or risk of preterm complications in subsequent pregnancies. Whether or not these increases are beneficial to female reproductive health and fertility remains unclear. However, a clear step change in cytokine expression was observed between the groups and warrants further investigation.
Abbreviations
G-CSF granulocyte colony-stimulating factor
IP interferon-inducible protein
PC principal component
PDGF platelet-derived growth factor
Acknowledgements
We are grateful to the Texas Tech University Health Sciences Center, Permian Basin, TX, and Ailena Mulkey, Jammie Holland, and Evangelina Santiago from Clinical Research Institute, TX for their excellent support on this study.
Authors’ contributions
GV consulted and obtained patient consent. GV and CRI nurses collected vaginal swab samples from all patients. KG performed the experiments. JG, KG, AS, NJM, AG, and GV analyzed & interpreted the data and contributed in manuscript writing as well as editing. All authors read and approved the final version of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.