Introduction
Endocrine-related cancer represents a broad area of major interest when considering the undesirable effect of hormones. This field of cancer with a hormonal background is often associated with common cancers such as breast and endometrial cancers, but also with less frequent neoplasms such as ovarian cancer. Colon cancer, or colorectal cancer to be more precise (CRC), however, is not mentioned as often, despite the high prevalence of this type of tumor, and its apparent gender pattern. In fact, data of 2023, indicates that CRC accounts for 8% of cancers in women in the US, making it the third most common cancer in both prevalence and mortality [1]. In addition, a similar impact has been found worldwide, where age-standardized incidence rates have been persistently lower in women [2]. Despite this background, the hormonal basis of CRC is an underrepresented topic in literature repositories compared to breast or endometrial cancer.
This review aims to update knowledge about the hormonal background of CRC, with an interest on estrogens. For that, we have reviewed the clinical studies supporting the association of CRC with estrogens, and have presented the solid biochemical data linking estrogen receptor beta (ER-β) with CRC tumors. Then, we summarize the main hypotheses on the pathways set in motion by the activation of the ER-β, and update the prognostic value of the receptor in clinical studies of patients with CRC.
Literature search
The PubMed database was searched from January 1, 2000 to December 6, 2023. No restrictions were imposed except for language, which was limited to English and Spanish. The search terms were "estrogen", "estrogen receptor" and "colon cancer". Inclusion criteria allowed for both experimental and clinical studies. Original articles, reviews and meta-analyses were included. Studies that did not include the two key search terms “estrogen” and “colon cancer”, or those focused on special populations, such as pregnant women or immunocompromised patients, were excluded. The search yielded 1,008 entries, which were reviewed by title by three of the coauthors (AM-T, RB-M, and AC-C), so that the screening wave left 254 titles. The list of articles was reduced to 71 after reading the abstract of each selected article. Detailed examination of these papers, plus that of the additional 13 retrieved by manual search of their references, yielded 40 papers, which have been included in the reference list of the present review.
Hormonal dependence of CRC: Clinical studies
The apparent gender profile of CRC has raised questions about a possible modulatory role of ovarian steroids. The question has been investigated from a dual perspective, the impact of menopausal hormone therapy (MHT) and the relationship between changes in endogenous estrogen exposure, in both cases considering the incidence or mortality linked to CRC.
Effect of MHT
Studies investigating the relationship of MHT with CRC started about 40 years ago. Early work by Nachtigall et al [3] already addressed the question in a small randomized double-blind study on a group of 84 pairs of postmenopausal women (treated and controls) who were followed for 10 years. No significant effect of MHT was observed, although the small number of participants severely limited the consistency of the results [3].
A milestone in the level of evidence is represented by the Women's Health Initiative (WHI) study, which confirmed a reduction in the risk of CRC in women who received MHT versus those assigned to placebo. The WHI study design included 2 main sub-studies depending on whether the women retained their uterus or had undergone hysterectomy. The first group received combined MHT, including conjugated equine estrogens plus a progestin (EPT), or placebo, while the second received estrogen alone (ET) or placebo. The numbers were sufficient to reveal any modulation of CRC risk, with 16,608 participants in the EPT study and 10,739 in the ET study. There was a 44% reduction in CRC incidence in the pool of women treated with EPT, hazard ratio (HR) = 0.56, 95% confidence interval (CI) 0.38-0.81 [4]; but no difference, HR = 1.08, 95% CI 0.75-1.55, was found between treatment and placebo in the ET study [5]. The apparent protection in the EPT study was not without question, as the intriguing finding was that tumors in the treatment arm were at a more advanced stage than those in women who received placebo.
The findings of the WHI study defined a key point in research regarding the association between ovarian hormones and CRC. A subsequent meta-analysis that included four randomized controlled trials (RCTs), eight cohort and eight case-control studies, concluded that there was protection associated with ever use of EPT, relative risk (RR) of 0.74, 95% CI 0.68-0.81, or ever use of ET, RR = 0.79, 95% CI 0.69-0.91 [6].
Effect of endogenous estrogens
From another perspective, the effect of ovarian hormones can also be highlighted when assessing endogenous levels and CRC risk. Interest has focused on estrogens, which circulate at very low but detectable levels in postmenopausal women. The hypothesis is that small differences in circulating hormones could translate into a distinct CRC risk. In this regard, a study on a subset of 1,203 women undergoing placebo in the WHI study confirmed that higher estradiol levels, defined by comparison between the 4th and 1st quartile, were associated with a reduction in CRC risk, odds ratio (OR) = 0.58, 95% CI 0.38-0.90.
Protection was also found for free estradiol and estrone, whereas no significant effect was observed for changes in circulating levels of progesterone. Sex hormone-binding globulin (SHBG) levels were associated with increased risk, OR = 2.30; 95% CI: 1.51-3.51 [7]. This finding further supports estrogenic action, since SHBG is known to bind free estradiol. A higher level of SHBG could be interpreted as a reduction in available estradiol and a reduced hormone action.
Consistent with these data, a meta-analysis of 5 studies evaluating the effect of hormone deprivation by ovariectomy found a 30% increased risk of CRC [8].
Long-term follow-up
Follow-up of women in the WHI study has intensified the doubts raised by the aforementioned lower incidence, but more advanced stage of tumors in women treated with EPT. Follow-up of women participating in the trial for an additional 6 years did not confirm a mortality advantage for women who were treated with EPT. Thus, despite a lower incidence during the years of the trial (mean 5.6 years), mortality was similar between the groups, and remained so until the mean 11.6 years of total follow-up [9].
In conclusion, current evidence suggests some protection of hormones, either MHT or endogenous hormones, for the incidence of CRC. However, this does not appear to provide any advantage in terms of mortality, either overall or CRC-related.
Biochemical background
Estrogen receptors and their function
Steroid hormones are regulators of the activation of their specific receptors. Cellular sensitivity to hormones, therefore, requires detectable expression of the receptors in the target tissue. In the case of CRC, and by extension colon epithelial cells, interest has focused on estrogen receptors (ER). This special attention has stemmed from findings in epidemiological studies, in which oncologic risk has been associated with the action of estrogens.
ERs are a component of the large family of steroid receptors. There are different ER species, mainly the intranuclear ones, associated with genomic actions, and the G protein-coupled estrogen receptor (GPER), which regulates rapid actions, not mediated by gene activation. Two main types of ERs mediate the genomic action of estrogens, the alpha (ER-α) and beta (ER-β) isoforms. Early work demonstrated that ER-α expression is low and ER-β expression is high in normal colonic epithelium [10]. Immunohistochemical studies have shown that ER-β expression accumulates in crypts and in the surface epithelium, along with other cell types such as endothelium or vascular smooth muscle cells of the vascular wall, among others [11]. In contrast, ER-β isoform expression is reduced in malignant CRC cells, suggesting that ER-β may have a protective effect, as previously demonstrated for breast and prostate tumors [12]. This finding was already observed some years ago in experimental models of tumorigenesis in mice, in which ER-β opposed the action of ER-⍺ [13]. Interest, therefore, has focused on ER-β.
The GPER receptor has been shown to favor colon cell proliferation and thus oppose the protection associated with ER-β. However, experimental conditions drastically modify the action of GPER, so the ultimate effect of activated GPER on tumorigenesis or tumor progression is not yet clear [14].
ER-β and inflammation
Inflammation is an important mechanism in the risk of developing CRC. This assertion is upheld by consistent evidence associating local inflammation and CRC in both experimental animals and in the human. In the latter, the association is supported by a wealth of clinical studies, which have shown a link of ulcerative colitis with higher risk for CRC [15,16]. Other chronic inflammatory conditions, such as those related to obesity or other inflammatory bowel diseases, have also been associated with an increased risk of CRC [17,18]. The impact of chronic inflammation has been additionally supported by the protective effect of anti-inflammatory treatment, like acetylsalicylic acid, which has been shown to reduce CRC risk in clinical trials [19].
These clinical observations are also supported by findings in several experimental models. Work with human cell lines of colon cancer experience a clear anti-tumoral effect, demonstrated at several levels, when they have been incorporated ER-β by transduction techniques. These observations have also been shown in tumor xenografts in immunodeficient mice [20,21].
A more sophisticated model is defined by mice with deletion of the ER-β gene, the so-called ER-β knockout (β-ERKO) mice. This model allows a detailed description of the changes imposed by the absence of ER-β in a living animal. Research with these mice has demonstrated the direct involvement of ER-β in the inflammatory role of tumor necrosis factor alpha (TNF-⍺). In this way, induction of tumors with oncogenic chemical compounds involved increased local expression of TNF-⍺, leading to mucosal inflammation and subsequent ulceration [22]. Similar inflammatory changes have also been confirmed with the loss of ER-β due to aging [23]. The details of the mechanisms involved in this regard are beginning to unravel, thanks to research models that have focused on the downstream action of TNF-⍺ mediated NFκB activation [24].
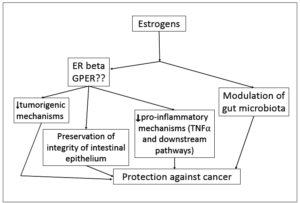
The contribution of microbiota is also an area of increasing interest. Histological analysis of the intestinal mucosa of β-ERKO mice has shown a deterioration of the mucosal architecture together with impaired integrity of the cell-to-cell junctions, all suggesting a degradation of epithelial permeability. This breakage of the intestinal barrier opens the way to a colonization of the mucosa by pro-inflammatory and tumorigenic species of the microbiota. Invasion of the epithelium and deeper layers of the colonic mucosa by enterotoxigenic and genotoxic microbial species, such as Escherichia coli or Bacteroides fragilis, has demonstrated a potential to disrupt antitumor immunity, favoring inflammatory conditions and promoting tumorigenesis [25,26]. The lipopolysaccharide associated endotoxemia favors the increase of NFκB signaling leading to the orchestration of inflammatory mechanisms. A summary of the estrogenic actions is shown in Figure 1.
Clinical evidence related with the ER-β
Expression of ER-β and prognosis
The value of ER-β as a prognostic biomarker in CRC has been paradoxically very sparsely investigated in clinical studies. Also, surprisingly, investigation of the clinical impact of ER-β agonists, whether natural such as genistein or equol, or synthetic such as ERB-26, has received even less attention.
The published literature is unanimous in associating ER-β expression with improved prognosis in CRC. In a study of 423 Chinese patients (190 women), with stage I-III CRC, ER-β expression was investigated. Patients were followed for a median of 86 months [27]. ER-β expression was associated with a higher 5-year survival rate, 84.3% vs. 63.9%, when comparing high vs. low expression levels.
Another study from Germany evaluated the overall survival, disease-specific survival and disease-free survival of 1,262 CRC patients (541 were women) according to the level of ER-β expression, which was investigated by immunohistochemistry. Compared with patients with high ER-β expression, those with negative ER-β suffered from more advanced cancer stages and greater tumor extension. Furthermore, ER-β was negativity associated with higher overall mortality (HR = 1.61; 95% CI: 1.09-2.40), CRC-specific mortality (HR = 1.54; 95% CI: 0.99-2.39) and worse disease-free survival (HR = 1.64; 95% CI: 1.23-3.36) [28].
A subsequent study in Finland focused on a group of 320 women with CRC. Primary CRC tumor samples were evaluated by immunohistochemistry with an antibody that detected positive signals for ER-β in 314 samples. Overall mortality was reduced by 50% in cases with higher ER-β expression (HR = 0.50; 95% CI: 0.11-0.52), and recurrence by 24% (HR = 0.24; 95% CI: 0.11-0.52) after adjusting for covariates [29].
A more recent study retrospectively investigated ER-β expression in 101 patients (41 women) operated on for high-risk CRC. ER-β expression was associated with a 79% reduction in the odds of a shorter disease-free survival [30].
In summary, the data from the reasonable number of patients investigated, totaling 2,106 in the 4 referenced studies, unanimously confirm a consistent association between ER-β expression and better prognosis, whatever the selected indicator.
Finally, a modern approach has taken into account the molecular pathways leading to CRC. Mismatch repair, phenotypically represented by microsatellite instability, is one of the mechanisms analyzed. Microsatellite instability is identified in about 15% of CRC cases. Some clinical evidence supports that patients with high microsatellite instability have a better prognosis than patients with low or stable microsatellite instability. A German meta-analysis found that the protective effect of MHT, with a 20% reduction in risk, was seen in the worse prognosis tumors in which microsatellite instability was stable [31]. Recent Japanese data of 116 postmenopausal women found an influence of age and of the side, left or right of the tumor, such that ER-β reduction, compared to areas of normal tissue in surgical specimens, was only observed in women younger than 70 years and with low mismatch repair. Further studies are needed to confirm and properly interpret these recent data [32,33].
Impact of clinical use of ER-β agonists
The potentialities of using compounds capable of stimulating ER-β as a more or less effective alternative to reduce the risk, or even treat CRC, have not been thoroughly investigated. Some attention has been given to natural compounds, such as some isoflavones, and in particular to those encompassed under the term "phytoestrogens", which have shown a preferential affinity for ER-β. In favor of promoting their use, a high number of epidemiological studies associate the consumption of isoflavones, in the form of natural soy in the diet, with a lower incidence of several types of cancer, among them CRC [34-36]. Based on these findings, isoflavones have been proposed mainly as a dietary supplement to reduce risk or, eventually, enhance the effect of pharmacological therapies when the disease is already a reality [37]. Support for the use of isoflavones in the clinical setting, however, has been proposed based only on experimental research studies which are very numerous, situation that must be acknowledged [38]. In contrast, clinical studies of sufficient quality have been occasional and hardly decisive. For example, one RCT comparing isoflavones administered as soy protein supplements failed to find any change in cell proliferation rates in participants with colon polyps [39].
The widespread use of isoflavones in CRC is not without some additional caveats. Phytoestrogens, such as genistein and others, have a higher affinity for ER-β, but this does not mean that the affinity for ER-⍺, although lower, is zero. This raises questions in those types of cancer where there are doubts as to whether ER-⍺ stimulation may involve any damage [40]. It is possible that, if used at higher doses in the context of supporting drug therapy of oncology patients, the effects may be detrimental. In addition, the full range of effects resulting from ER-β stimulation is not fully understood [34].
Conclusion
There is a strong background supporting a protective action of ER-β on CRC. Epidemiological data have reported a reduction in the prevalence of these tumors in women worldwide. This is coupled with clinical studies, both observational and RCTs, which have found a protective action of estrogens. The major role of ER-β, rather than ER-⍺ or GPER, is derived from functional pathology studies, which describe a decreased expression of ER-β in CRC samples compared to normal tissue. In addition, there is a morphological alteration of the intestinal epithelium, which breaks the integrity of the mucosa. Different experimental models show ER-β involvement in various malignancy features, such as mitosis or evident xenograft growth in live animals. The value of ER-β as prognostic indicators in clinical cohorts of patients suffering from CRC has been confirmed in clinical studies. However, interventional studies in clinical trials are lacking, so the role of ER-β in this regard remains to be proven.
Conflict of interest
The authors declare having no conflicts of interest.