Introduction
Endometriosis is a prevalent condition which occurs in an estimated 1 out of 10 women during their reproductive lifespan [1]. Worldwide, this translates to 190 million women with endometriosis. The most commonly occurring symptom is dysmenorrhea, but clinical presentation and disease severity vary widely [1]. Endometriosis usually involves the pelvic organs including the peritoneum, the genital organs, ligaments, bowels and/or urinary system. Less commonly, endometriosis may occur extra-abdominally in the thoracic cavity, abdominal wall, as well as in the perineum. In this case report we present a patient with repeated perineal endometriosis recurrence in an episiotomy scar, with a potential pathophysiological role of von Willebrand Disease (vWD).
Case report
We present a 34-year-old patient, with type 1 vWD, who visited the outpatient clinic of her local hospital in 2021 with a painful swelling in her episiotomy scar. She was multiparous after two vaginal deliveries. Her first delivery occurred in 2013 with an episiotomy, and was complicated by postpartum hemorrhage of 3,500 mL based on uterine atony and episiotomy wound bleeding, with vWD as predisposing factor. Desmopressin was administered before her second delivery, which occurred in 2015 without complications. After her last delivery the patient did not use any form of hormonal contraception. The swelling was treated through incision and drainage, releasing a dark-brown fluid, which was not sent in for analysis.
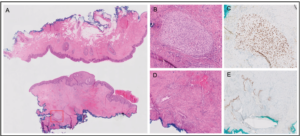
Two months later, the painful swelling re-emerged. Because of her comorbid vWD, she was referred to our tertiary hospital. At physical examination, a blue-colored swelling at the site of her episiotomy scar was observed, with a diameter of 1-2 cm. Due to the severity of her pain, she was directly admitted to the operating theater for drainage of the swelling. Based on clinical suspicion of endometriosis, the lesion was excised with a margin of neighboring tissue under general anesthesia. Remaining tissue was electrocoagulated and the defect was closed primarily. Intravenous desmopressin, a vasopressin analog, was administered pre- and postoperatively as a hemostatic agent, inducing the production of von Willebrand factor. Endometriosis was diagnosed after histopathological analysis (Figure 1), and hormonal treatment with a combined oral contraceptive (COC) pill was started.
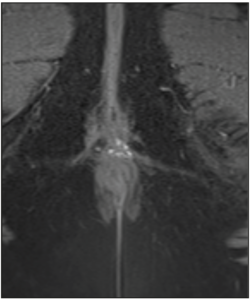
Six months after initial presentation, while still using COC continuously, the patient returned with a recurrence of the painful swelling in her episiotomy scar. A MRI was performed, showing a T2 non-well-demarcated soft tissue collection of 11 x 13 x 6 mm between vulva and anus. The T1 imaging revealed foci of high intensity, indicative of blood, therefore confirming the suspicion of a perineal endometriosis recurrence (Figure 2). A second re-excision, containing a margin of 5-10 mm of surrounding tissue was performed. The tissue of the wound bed was treated with plasma argon energy to further decrease the risk of recurrence. In addition to the desmopressin administered pre- and postoperatively, TachoSil fibrin sealant patches were used for local hemostasis. Histological analysis supported again the diagnosis of endometriosis.
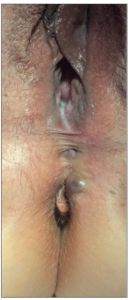
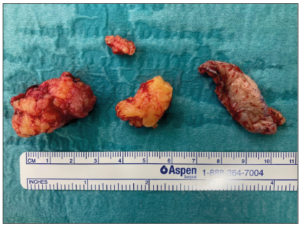
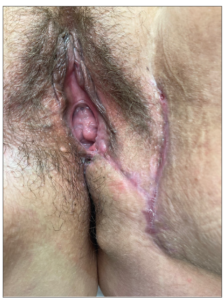
Sixteen months after initial presentation, and 10 months after the second excision, the patient presented with a renewed painful swelling at the site of her episiotomy scar, which had appeared at the end of a hormone-free week (which she took every several months to prevent prolonged spotting complaints while using COCs). The resulting pain was such that the patient was unable to sit and had severe pain during defecation. Upon examination there were two blue-colored lesions with a diameter of 4-5 mm, located respectively right above the posterior commissure of the labia and ventrally of the anal sphincter, which were extremely sensitive to touch (Figure 3). A MRI was performed which revealed a perineal endometriosis lesion, adjacent to the sphincter complex but without signs of muscular infiltration. A second re-excision was performed, during which approximately 5 cm3 of endometriosis tissue (Figure 4) with a margin of 10 mm was removed and the remaining field of tissue was treated with argon plasma energy. The perineum was reconstructed using a pedicled, partly de-epithelialized, lotus petal flap, based on internal pudendal artery perforators from the left groin area. Prophylactic measures to prevent blood loss were undertaken again, for which reason the patient was admitted until 5 days post-surgery. The hormonal treatment was switched to dienogest 2 mg continuously. At six weeks follow-up post-surgery the surgical site was healing adequately and without complications (Figure 5).
Discussion
Although the occurrence of endometriosis in an episiotomy scar has been described previously [2-4], it rarely presents at this location, with an estimated prevalence of 0.01% [5]. The exact pathophysiology of endometriosis in a perineal scar remains unknown, but most consensus exists on the theory of transplantation and implantation of endometrial cells towards the perineum during vaginal delivery [5].
The ‘classic diagnostic triad of perineal endometriosis’ has been described elsewhere [3] and additionally applies to our patient: 1. an episiotomy of past perineal tear during vaginal delivery; 2. a tender mass or nodule at the perineal lesion; and 3. progressive (cyclic) perineal pain. Physical examination should include the examination of the painful nodule, as well as the anorectal region and rectovaginal septum. Both ultrasound and MRI can be used as diagnostic modalities, however MRI is the preferred imaging technique for precise measurement of the lesion size as well as an assessment of the involvement of the rectum and the anal sphincter complex. Furthermore, with endometriosis in the differential diagnosis, examination should include the identification of endometriosis in the broader pelvic and intra-abdominal regions. In a cohort of 14 women with perineal endometriosis, 3 women (21%) were found to have pelvic endometriosis [6]. In this cohort there was a recurrence rate of 14%, with most presentations within a year of initial treatment [6].
The treatment of perineal scar endometriosis is primarily surgical. A local excision of the nodule or lesions should be undertaken with up to 10 mm margin of healthy tissue, as an incomplete resection can lead to lesion recurrence. Subsequent treatment with plasma argon energy is additionally preferred to minimize the risk of recurrence [2,7]. The excised tissue should be examined histologically to confirm the diagnosis of endometriosis as well as ruling out the possibility of malignancy, specifically in recurrent disease, as malignant evolvement of perineal endometriosis has been described previously [8]. With larger perineal resections, a collaboration with a reconstructive surgeon seems advisable, to maximize postoperative cosmetic and functional outcomes and to minimize wound healing complications.
vWD is the most common bleeding disorder, affecting up to 1% of the population based on abnormal laboratory parameters alone [9]. This inherited condition occurs equally in men and women as well as across all races and ethnicities. However, women may be more symptomatic due to heavy menstrual bleeding periods. A combination of blood tests was used to diagnose vWD, including vWF antigen, as well as a platelet binding assay to differentiate between qualitative and quantitative defects. The three recurrences, despite adequate surgical measures and continuous COC treatment, are striking in this case. In a study conducted by the American Centers for Disease Control (CDC) among 102 women with vWD, 30% of the women were found to have endometriosis, in comparison to 13% in the control group (p=0.005) [10]. Moreover, a recent case reported recurrent ovarian endometriomas in a 17-year-old vWD patient using continuous hormonal medication [11], similar to our patient who also had recurrences despite hormonal treatment. While the literature is still scarce, the increased prevalence and seemingly more severe or treatment-resistant presentation of endometriosis suggest that the hematological hallmarks of vWD may directly influence the pathophysiology of endometriosis. It is possible that the increased bleeding tendency in vWD increased the risk of endometrial cell implantation and may have exacerbated the activation of existing endometriosis lesions. Interestingly, however, a recent Mendelian randomization analysis demonstrated a causal relationship between the coagulation factors ADAMTS13 and vWF and the risk of endometriosis, with higher vWF antigen levels predisposing to the endometriosis development [12]. Consistent with these findings, ectopic endometrial cells in patients with adenomyosis generally appear to exhibit more angiogenic properties and increased vWF antigen activity [13].
Taken together, there is a clear link between both increased and impaired coagulation and endometriosis, further highlighting the complexity of the disease and the impact of comorbidity on its pathophysiology. Although there is no evidence to date to support the treatment of coagulation disorders as a means of improving the symptoms of endometriosis, awareness of such problems can be helpful in tailoring endometriosis treatment.
In conclusion, while unlikely, endometriosis can occur in the perineum and can be included in the differential diagnosis of perineal swelling in women. Patients with vWD may present with more severe symptoms and/or recurring disease, increasing complexity and challenging clinicians to find an appropriate choice of therapy which has to be tailored to the needs of the patient.
Conflicts of interest
The authors declare having no conflicts of interest.
Data availability statement
The data presented in this paper are not publicly available as they pertain to personal medical results. Any queries can be presented to the authors.
Ethics statement
The patient in question gave written informed consent for inclusion of her data in this case report.