Introduction
The demand for up-to-date information in the palm of our hand and the exponential advancement of technology within the last decade has caused the modern world to become dependent on cellular telephones, specifically smart phones. As of 2020, 97% of Americans owned a cellular telephone and 85% of Americans owned a smartphone [1]. Nearly 44% of cellular telephone owners sleep with their phones within an arm’s reach away to prevent missing any phone calls, text messages, or any other updates [1]. Half of Americans are exposed 24-hours/day to a cellular telephone in close proximity, which may be putting them at risk for the potentially harmful effects of radiofrequency radiation exposure. In 2012, we presented a study that brought some of these potential harmful effects to light and showed novel evidence of neuropathology due to fetal cellular telephone radiation exposure. The study demonstrated that fetal exposure to 800-1,900 MHz-rated radiofrequency radiation from cellular telephones leads to behavioral and neurophysiological alterations, specifically, impaired memory, hyperactivity, and decreased anxiety, which indicates that in-utero exposure to radiofrequency radiation is a potential cause of neurobehavioral disorders such as attention deficit hyperactivity disorder (ADHD) [2]. The study also suggested that one mechanism by which fetal cellular telephone radiation exposure leads to an increased prevalence of neurobehavioral disorders is through the impairment of glutamatergic synaptic transmission onto pyramidal cells in the prefrontal cortex [2].
While we previously demonstrated that fetal exposure to radiofrequency radiation from cellular telephones causes behavioral changes in mice, the effect on development and aging remained unknown. A major unresolved issue in aging research is the unclear distinction between normal aging, consisting of a moderate decline in cognitive performance, and pathological or premature aging, consisting of a mild or severe decline in cognitive performance. One factor that may contribute to premature aging is the integrity of the cholinergic system. Cholinergic basal forebrain neurons and their associated projections into the cortex show increased susceptibility to aging and cognitive decline is associated with loss of neuronal nicotinic acetylcholine receptor (nAChR) function [3,4]. A study that utilized β2-/- mice, who have the gene encoding the β2 subunit of the nAChR genetically deleted, showed that high-affinity nicotinic signaling plays a region-specific role both on morphogenesis and maintenance of layer V pyramidal neurons by enhancing age-related decline in neuronal structure [5]. The mice from our previous study which were exposed as a fetus to radiofrequency radiation from cellular telephones had dose-responsive impaired glutamatergic synaptic transmission onto these same layer V pyramidal neurons of the prefrontal cortex [2]. Thus, in-utero exposure to cellular telephone radiation may be a factor leading to premature aging through this proposed mechanism. We re-evaluated these mice at 18 months of age to determine if there were further deleterious effects worsened with aging.
We also examined the effect of fetal cellular telephone exposure on another parameter that we had not previously measured: reproduction. Both environmental and genetic factors are thought to program reproductive performance in mice [6-9]. There are many specific, quantitative trait loci, that are involved in the regulation of reproductive performance, which may explain the differences in litter size, viability, and birth weight between different strains of mice [10-13]. Various environmental factors such as temperature, humidity, ammonia, carbon dioxide, light, sound, and type of cage ventilation system can also affect breeding performance [14]. The birthing data of the mice involved in this study were investigated here to see if there was any effect of fetal exposure to cell phones on subsequent reproductive performance.
In addition to the duration of neurologic effects, we also investigated whether exposure after the fetal period would have similar effects. To determine whether post-natal radiofrequency radiation exposure from cell phones has similar neurodevelopmental and behavioral effects as in fetal exposure, a group of mice were separately exposed to the same experimental parameters postnatally and similar methods were used to analyze memory and behavior.
Methods
In Utero Exposure
A total of 27 breeding cages were set-up each containing 3 CD-1 female mice and 1 CD-1 male mouse (13 experimental cages and 14 control cages). Each experimental cage was equipped with a muted and silenced 800–1,900 MHz cellular phone with a specific absorption rate (SAR) of 1.6 watts/kg placed over the feeding bottle area at a distance of 4.5–22.3 cm from the mice. The cellular phones were then placed on an active call for 24 hours per day and the 39 experimental female mice were exposed throughout gestation (days 1–17). Each control cage was equipped with a deactivated phone and was kept under the same conditions. To assure equal exposure time independent of the variable length of gestation (18–20 days), at the end of day 17 all phones were removed. On day 18, all female mice were separated and placed in their own cage yielding a total of 39 exposed pregnant females and 42 unexposed pregnant females. Throughout gestation, both the control and experimental mice were fed and given water ad libitum. The mice were maintained on a 12 hour light/dark cycle (07:00 am on) and all procedures were approved by the Yale University Animal Care and Use Committee [2].
Breeding
Reproductive performance was assessed by taking the G1 male and female offspring from the CD1 wild type mice that were exposed to cellular phone radiation during breeding and mating them with wild type mice. Reproductive performance of the experimental offspring was subsequently measured utilizing the following 6 outcome criteria: gestation and birth, viability of the pups, lactation, survival success, production, and weight. Gestation refers to the total number of pups and births refer to the number of pups born alive. The viability of the pups was determined by the number of mice alive on day 4, and lactation was determined by the number of mice alive on day 12. The survival success rate was interpreted as the number of mice weaned. The production was determined by the number of mice weaned compared to the number of mice expected from breeding [2].
Aging mice
The effect of aging was evaluated using a standard object recognition memory test at 3 and 18 months of age after fetal exposure. A total of 52 pups were tested (26 experimental mice and 26 control mice.) Over two learning days (Day 1 and 2) and one test day (Day 3). Four opaque exploration chambers were set-up in the exam room at a luminosity of 420–440 Lux, and the mice were placed in the testing room and allowed 1 hour to acclimate to the light. On Day 1 two identical objects were placed in each of the four chambers and a single mouse was placed in each chamber to explore the two identical objects for 15 minutes. Before repeating the experiment, the objects and the chambers were cleaned thoroughly with a detergent solution to remove any scent or odors. On Day 3 a video camera was placed over all 4 chambers and the objects were rearranged so that each chamber had one familiar object and one novel object. The mice were then allowed to explore both objects and were filmed for 5 minutes. Upon completing the experiment, 3 observers, blinded to the treatment regimen, viewed the footage to determine the time spent exploring the novel object. Exploration of the new object was defined as sniffing at less than 1 cm. A preference index was then calculated by dividing the time spent exploring the new object by the total exploration time multiplied by one hundred. A decrease in preference index indicated diminished memory. The percent time spent idle - not exploring either of the objects was also calculated in order to ensure that our findings are in fact due to memory deficits and not distractibility or hyperactivity [2].
Postnatal exposure
To measure the effects of postnatal cellular telephone radiation on behavior, the experimental parameters were as follows. Sixty-six pups were continuously exposed postnatally from birth for 28 days to a muted and silenced 800-1,900 MHz cellular phone with a SAR of 1.6 watts/kg. The phones were muted and placed on a continuous active call and positioned over the feeding bottle area at a distance of 5-25 cm from the mice pups. Twenty-seven control pups were exposed postnatally to a deactivated phone and kept under the same conditions.
To evaluate the behavior, pups were subjected to a light-dark box test using a light-dark box constructed of black and white Plexiglass (45×27×27 cm). The dark compartment (18×27 cm) was made of black Plexiglass with a black Plexiglass cover and the light compartment (27×27 cm) was made of white Plexiglass and remained open. The light compartment was kept at a luminosity of 420–440 Lux. An opening (7.5×7.5 cm) was located in the wall between the two chambers allowing free access between the light and dark compartments. A video camera was then placed over the box for filming. The mice were then placed in the testing room and allowed 1 hour to acclimate to the light. A single mouse was then placed in the light chamber and was allowed to explore the box for 5 minutes while being filmed. Before repeating the experiment, the chambers were cleaned thoroughly with a detergent solution to remove any scent or odors. Three observers, blinded to the treatment regimen, then viewed the footage and recorded the total time spent in the dark as well as the total number of transitions. This data was then interpreted as described in the text to analyze anxiety and hyperactivity. The Step-Down Assay was performed to determine fearful behavior by placing a mouse gently on a platform (96 well plate) and recording the time on the platform. The timer was stopped once the mouse stepped off the platform with all four paws. Before repeating the experiment, the platform was cleaned thoroughly with a detergent solution to remove any scent or odors [2].
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 7.0 software. Memory and behavioural data were analyzed by an unpaired T -test. A p value < 0.05 was considered as statistically significant.
Results
Fetal exposure and neurocognitive aging
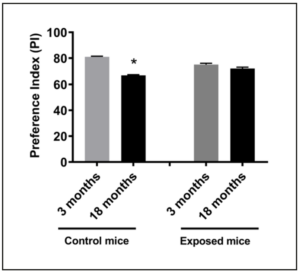
In 2012, we demonstrated that fetal exposure to cellular telephone radiofrequency radiation leads to behavioral and neurophysiological alterations including impaired memory and hyperactivity, but the effect on cognitive decline and aging has remained unknown. In order to evaluate this, twenty-six exposed offspring and twenty-six control offspring underwent the standard object recognition memory test at three months of age and eighteen months of age. Three blind observers evaluated the test and preference indexes were calculated based on the observer’s recorded times. The mean preference index at three months of age for the control mice and the exposed mice was 80.5% and 73.4%, respectively (Figure 1). Comparing controlled versus exposed mice, these indexes indicate an adverse effect on memory due to in-utero cellular phone exposure (p<0.05). The mean preference index at eighteen months of age for the control mice and the exposed mice was 66.7% and 71.4%, respectively (Figure 1). The control mice had a significant decrease in mean preference index between three months and eighteen months of age (p=0.0017) attributed, most likely, to a normal decline of memory with aging. However, the exposed mice did not have a statistically significant difference in preference index from three months of age and eighteen months of age, indicating the initial deficit at three months may have been due to early damage which did not continue to progress with aging. Thus, exposure to cellular telephones in-utero may lead to accelerated deterioration in memory that normally occurs during the aging process.
Fetal exposure and reproduction
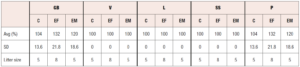
To determine the effect of fetal exposure to cellular phones on the reproductive performance of mice, CD1 wild type mice were bred in which the females were exposed to cellular phone radiofrequency throughout gestation and the G1 offspring were evaluated. The reproductive performance was evaluated using the following outcome measures: gestation and birth (GB), viability of the pups (V), lactation (L), survival success (SS), production (P), and weight (W). Gestation and birth refer to the total number of pups and number of pups born alive. The viability of the pups was determined by the number of mice alive on day 4 and lactation was determined by the number of mice alive on day 12. The survival success rate was interpreted as the number of mice weaned. The production was determined by the number of mice weaned compared to the number of mice expected from breeding. As shown in Table 1, there was no significant difference in gestation and birth, viability, lactation, survival success or production between G1 male or female mice when compared to controls. In-utero exposure of mice to cellular phone radiofrequency does not appear to effect overall reproductive parameters such as litter size, viability, lactation, survival success, birth weight or production.
Postnatal exposure
Our previous study [2] demonstrated that fetal exposure to cellular telephone radiofrequency could affect neurodevelopment and behavior. To explore if these same effects can be seen in mice exposed to cellular telephone radiofrequency postnatally we evaluated the postnatal effects on behavior of an 800-1,900 MHz-rated device with a SAR level of 1.6 watts/kg. The SAR is a measure of tissue radiation exposure and the United States has set a SAR limit of 1.6 watts/kg [3]. Sixty-six pups were continuously exposed postnatally for 28 days to a muted and silenced 800-1,900 MHz-rated cellular phone with a SAR of 1.6 watts/kg. The phones were placed on an active call for 24 hours per day and were positioned over the feeding bottle area of the cage. Due to the fixed position of the phones, the distance between the phone and the mice varied between a minimum distance of 5 cm and a maximum distance of 25 cm. Twenty-seven pups acted as our control group and were exposed postnatally to a deactivated phone emitting no radiofrequency radiation and kept under the same conditions.
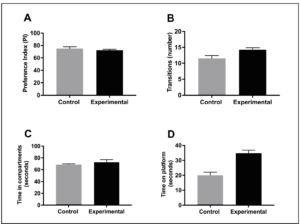
The pups (66 exposed and 27 not exposed) were investigated using the same methods from our initial study which included: the standard object recognition memory test, the light/dark box test, and the step-down assay at 8 weeks of age. The standard object recognition memory test is performed by first allowing the mice to explore two identical objects for 15 minutes a day for two days. On the third day, a novel object replaces one of the familiar objects and the mice are filmed for 5 minutes exploring the novel object and the familiar object. Three blind observers watched the film and recorded the exploration time for the novel and familiar objects. Then, a preference index is determined by dividing the time spent exploring the new object by the time spent exploring both the new and familiar objects, multiplied by one hundred. Thus, a decrease in preference index indicates diminished memory. The mean preference index in the exposed group was 71.6% as compared to control group (73.2%), indicating no impairment in memory in the exposure group (Figure 2A). The light/dark box test assesses hyperactivity and anxiety by utilizing a mouse’s natural aversion to bright light. The box contains two compartments: one white compartment that was illuminated with bright light and one black compartment that was kept dark. The number of transitions between the two compartments determines the locomotion, and in turn, the hyperactivity of the mice. Anxious behavior is determined by recording the actual time spent in each compartment. Each mouse was placed in the light/dark box one at a time and recorded for 5 minutes. Similar to the standard object recognition memory test, the film was reviewed by three blind observers and the number of transitions between compartments and the total time spent in each compartment was measured. The mean number of light/dark compartment transitions in the exposed group was 13.6 as compared to 11.3 in the control group (Figure 2B). The difference in the mean number of transitions was not statistically significant (p= 0.181) and confirms no signs of hyperactive behavior in either group. The total time spent in the compartments also displayed minimal variation between the two groups with 71.5 seconds in the exposed group and 69.6 seconds in the control group showing no statistical significance (p= 0.228) (Figure 2C). Thus, anxious behavior was not demonstrated by either group. Lastly, the step-down assay was employed to assess fear of exploring the environment. The test is performed by recording the amount of time a mouse spends on a standard platform before entering the environment. The greater the time spent on the platform, the more fearful the mouse is. Exposed mice showed no significant difference in time spent on the platform, 31.2 seconds, when compared to the control mice, 19.6 seconds (p= 0.163) (Figure 2D). These results indicate that mice exposed postnatally to cellular telephone radiofrequency radiation demonstrate no signs of impaired memory, hyperactivity, anxiety, or fear.
Discussion
In 2012, we demonstrated that fetal exposure to 800-1900 MHz rated radiofrequency radiation from cellular telephones leads to behavioral and neurophysiological alterations that persists into adulthood. The mice that were exposed in-utero had impaired memory, were hyperactive, and had decreased anxiety, indicating that radiofrequency radiation is a potential cause of neurobehavioral disorders. Furthermore, we proposed a possible mechanism by which cellular telephone radiation may lead to an increased prevalence of neurobehavioral disorders by demonstrating that impairment of glutamatergic synaptic transmission onto pyramidal cells in the prefrontal cortex was associated with these behavioral changes. In the present study, we investigated the effects of cellular telephone radiation on fertility and reproductive performance, the effects of cellular telephone radiation exposure postnatally, and the role in-utero radiofrequency radiation emitted from cellular telephones may play in age related cognitive decline.
Fetal exposure of mice to cellular phone radiofrequency does not appear to affect overall reproductive performance. There was no difference between the offspring of control mice that were not exposed to any radiofrequency radiation and the offspring of experimental mice that were exposed to in-utero radiofrequency radiation in the following categories: birth and gestation, viability, lactation, survival success, and production. The preserved fertility suggests that the hypothalamus, pituitary and ovaries were likely unaffected.
Few studies have been performed evaluating the effects of radiofrequency radiation on female fertility or reproductive performance, but a plethora of animal studies exist showing how radiofrequency radiation affects the male sperm. Mailankot et al exposed male rats to 1 hour per day to an active cellular phone call for a consecutive 28 days and found a significant decrease in sperm movement and activity when compared to a non-exposed group of rats [14]. Additionally, Aitken et al performed a study that exposed male mice to 900 MHz of radiofrequency radiation at a SAR of 0.09 watts/kg for 7 consecutive days for 12 hours a day showing statistically significant damage to both the mitochondrial genome (p < 0.05) and cell nucleus (p < 0.01) [5]. Although there is considerable evidence based on prior studies that radiofrequency radiation emitted from cell phones damages sperm and is associated with male infertility, there is little evidence suggesting any correlation between cell phone radiation and female infertility. Our study clearly showed that in-utero cell phone radiation does not affect female fertility or reproductive performance. Adult exposure may differ in its effects. Chen et al performed a study that investigated the effects of 935 MHz electromagnetic radiation on fertilization and subsequent embryonic development in mice and found that mid- and high-intensity electromagnetic radiation of 935 MHz can reduce the fertilization rate in mice, and reduce the blastulation rate, thus reducing the possibility of embryo implantation [3]. The significant difference in results between our study and Chen’s is likely attributable to the effect in-utero vs adult cellular telephone radiation exposures on female fertility and reproductive performance.
In 2012, our study clearly showed that fetal radiofrequency radiation caused behavioral and neurophysiological alterations in the offspring of mice that persisted into adulthood. In the present study, we used the same parameters and methods in order to see if the same type of exposure to offspring postnatally would yield similar results. As discussed and shown above, there was no significant difference between control and experimental mice that were exposed to radiofrequency radiation postnatally regarding memory, hyperactivity, anxiety, or fear. These results further validate our study from 2012 that claim fetal, as opposed to postnatal, exposure to radiofrequency radiation causes behavioral and neurophysiological changes, such as those seen in ADHD.
Another prominent finding in the present study was identified when comparing the preference index, a means of assessing memory and cognitive function, between control mice to mice that were exposed in-utero to radiofrequency radiation at three months and eighteen months of age. The control mice had a significant decrease in preference index, as shown in Figure 2, between three and eighteen months which can be attributed to normal decline of memory with aging. Nevertheless, the exposed mice did not have a statistically significant difference in preference index from three months to eighteen months. Instead they began with an initial deficit at three months of age that we believe was due to early and permanent damage, an effect that can be considered premature aging. Premature aging has been associated with other disease processes. For example, Cohen et al conducted a study suggesting that HIV causes premature cognitive and brain aging through direct damage from the virus as well as indirectly through increased risk of cardiovascular disease, chronic drug use, and potentially toxic long-term antiretroviral use [4]. Another study investigated the implication that hypervitaminosis D3 promotes premature aging through the regulation of 1-alpha hydroxylase expression. Using vitamin D3 receptor knockout mice, the authors concluded that vitamin D3 receptor genetic ablation promotes premature aging in mice and that vitamin D3 homeostasis regulates physiological aging [15]. Pathological causes such as these are well documented to lead to premature aging, but, environmental factors have now been shown to also possibly play a role. Two main mechanisms for the cause of premature aging have been identified in mice. The first being the klotho theory. The klotho gene encodes a single-pass transmembrane protein that forms a complex with multiple fibroblast growth factor (FGF) receptors and functions as an obligatory co-receptor for FGF23, which is a bone-derived hormone that induces negative phosphate balance. Defects in either klotho or FGF23 gene expression can cause a premature-aging syndrome in mice, as well as phosphate retention by loss of regulation of growth factors such as insulin, IGF-1, and the Wnt signaling pathway and loss of the klotho protein’s ability to protect cells and tissues from oxidative stress [16]. The other popular proposed mechanism for premature aging was discussed earlier in the introduction of this paper and is correlated with our own results. A study by Konsolaki et al showed that premature aging may be caused by a loss of integrity in the cholinergic system [17]. Further, increased susceptibility to aging and cognitive decline is associated with loss of nAChR function in the cholinergic basal neurons and their associated projections into the cortex [18,19]. The study utilized β2-/- mice who had the gene encoding for the β2 subunit of the nAChR genetically knocked out to show that high-affinity nicotinic signaling plays a region-specific role both on morphogenesis and maintenance of layer V pyramidal neurons by enhancing age-related decline in neuronal structure [17]. In 2012, we showed that in-utero radiofrequency radiation from cellular telephones had dose-responsive impaired glutamatergic synaptic transmission onto these same layer V pyramidal neurons of the prefrontal cortex [2]. Based on our results from 2012 and the mechanism of premature aging proposed by Konsolaki in 2016 [17], the initial deficit we observed in our exposed mice when compared to our control mice (Figure 1) may be premature aging or permanent cognitive impairment due to in-utero radiofrequency radiation exposure to cellular telephones.
In summary, the present study builds upon our prior study in 2012 that demonstrated that fetal exposure to 800-1,900 MHz rated radiofrequency radiation from cellular telephones leads to behavioral and neurophysiological alterations that persist into adulthood. Here we tested mice exposed to radiofrequency radiation from fetal into their late adulthood and discovered a significant decrease in preference index, a measurement of memory and cognitive function, at an early age that remained decreased throughout their lifespan that may have contributed to permanent damage or premature aging. The control mice had a higher mean preference index at an early age but had a significant decrease by late age that we attributed to normal decline due to aging. We also investigated reproductive performance of mice exposed to radiofrequency radiation and concluded that fetal exposure of mice to cellular phone radiofrequency does not affect overall reproductive performance. Additionally, we explored the possible effects of offspring exposure to radiofrequency radiation postnatally and found no signs of impaired memory, hyperactivity, anxiety of fear. Finally, fetal radiofrequency radiation exposure results in premature and persistent neourobehavioral aging. Postnatal exposure had no effect in our experimental paradigm.
Funding
This research was supported by NIH grant: U54 HD052668 grant.
Conflicts of interest
Authors declare having no conflicts of interest.