Introduction
Cesarean scar pregnancy (CSP) is a rare form of ectopic pregnancy implanted in the region of a previous cesarean section scar at the level of the uterine isthmus, where the myometrium is narrower and thinner [1]. The prevalence of CSP strongly depends on the rate of cesarean deliveries, which is estimated to have reached its highest peak in 2009 (32.9%) after increasing every year since 1996 (20.7%) [2,3]. Although the incidence of CSP is extremely difficult to estimate, Seow et al. suggested that it is around 1:2216 of all pregnancies. Moreover, CSP seems to have a frequency of 0.15% in women who had at least one previous cesarean section and to account for 6.1% of total ectopic pregnancies in the same population [4].
The literature background shows that a history of cesarean sections is the main risk factor for CSP [5]; furthermore, the risk of developing this pathology increases proportionally to the number of cesarean deliveries undertaken, and is also related to the extension of the scar surface [6].
However, other factors have been recognized as predisposing to CSP, such as manual removal of the placenta, in vitro fertilization, a history of curettage and myomectomy, as well as the presence of adenomyosis [7]. Although the mechanism of implantation in the cesarean scar is still not clear, some authors have proposed that it may depend on abnormal myometrium invasion between the previous cesarean section scar and the endometrium, through a small canal [8]. This mechanism seems to be similar to that leading to placenta accreta, which is one of the main complications associated with scar pregnancy [9]. As regards the symptoms of CSP, the main manifestation is mild and painless vaginal bleeding; however, an association with pain is reported by 16% of patients. On the other hand, 9% of patients report abdominal pain alone as the presenting symptom; conversely, incidental diagnoses in asymptomatic women have also been described in the literature [10].
Evolution of CSP beyond the early weeks of gestation is associated with a high risk of life-threatening complications such as massive hemorrhage and uterine rupture, which might lead to hysterectomy with catastrophic reproductive consequences [11]. With regard to the diagnosis, transvaginal ultrasound plays a fundamental role in the early detection of CSP [1], with a reported sensitivity of 86.4% [10]. On the other hand, magnetic resonance imaging may help to define the implantation site and myometrium infiltration level and may thus guide appropriate management choices [12]. Several approaches to the treatment of CSP have been proposed over the last 10 years; among them, uterine artery embolization (UAE) is a valuable option, allowing preservation of the patient’s fertility with a minor risk of complications. Our study reports the case of a CSP pregnancy successfully treated with UAE and proposes a follow-up “model” suitable for cases similar to ours.
Case presentation
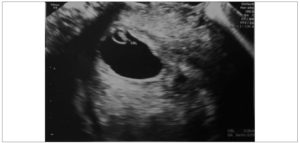
We describe the case of a 35-year-old woman who accessed our emergency obstetrics department with moderate pelvic pain. Her personal history included an urgent laparotomy for acute appendicitis during childhood, while her obstetric history was notable for two cesarean sections, 11 and 8 years before, respectively. Moreover, she reported having stopped using oral contraception almost 3 months prior to accessing our hospital, and that her last period had occurred about 40 days back. Obstetric examination revealed lower pelvic pain which increased during the speculum examination; no vaginal or uterine bleeding was noted. Blood tests showed a high serum B-hCG level (29991 mUl/ml). The patient was subsequently evaluated using transvaginal ultrasound, which revealed the presence of a 20-mm gestational sac implanted in the isthmic area within the previous cesarean section scar; the sac contained an embryo with a crown-rump length (CRL) of 4 mm (gestational age: 6 weeks plus 1 day) and with cardiac activity (Figure 1). Moreover, a hematoma area not producing vaginal bleeding was documented. Due to the patient’s hemodynamic stability and normal kidney and liver parameters, bilateral UAE with methotrexate (MTX) was proposed to the patient after she refused expectant management. The UAE was performed, puncturing the femoral artery after identification of the middle part of the right inguinal ligament. A 2.7 Fr. catheter was used to obtain digital selective angiography of the bilateral internal iliac arteries and super-selective angiography of the bilateral uterine arteries. The image showed thickening of the uterine arteries, especially in their ascendant portion, and hypervascular area near the gestational sac. MTX was injected into the uterine arteries (25 mg for each); this was followed by the embolization of the same vessels with microspheres of 700 micron. After removal of the catheter, the access point was bandaged and compressed for 15 minutes. Antibiotic therapy with ceftriaxone 2g (i.m.) and metronidazole 500mg (os) was administered during the surgical procedure and continued post-intervention. Moreover, an analgesic protocol was also applied during the procedure (diclofenac 100 mg per rectum, paracetamol 1g i.v. and morphine sulphate 10 mg i.m.). Administration of analgesic therapy was continued postoperatively with paracetamol 1g (os or i.v.) and ibuprofen 400 mg (os). No complications were reported during or after the procedure; indeed, hemodynamic stability, with regular blood pressure parameters and cardiac frequency, was maintained. No episodes of fever were registered.
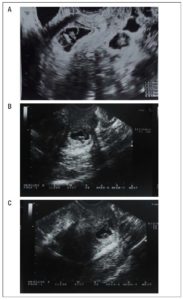
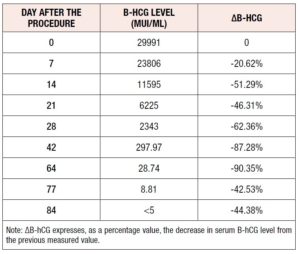
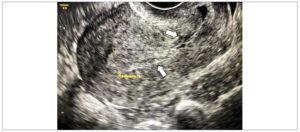
The patient was hospitalized in our department for one week: a transvaginal ultrasound was performed at day three from the procedure and showed a dysmorphic gestational sac of 28 mm x 15 mm containing a dysmorphic embryo (CRL=7 mm) without cardiac activity surrounded by a hematoma area (11 mm x 10 mm) (Figure 2.A). After the patient’s discharge, a follow-up with transvaginal ultrasound was scheduled every 14 days. Figure 2 B-C shows the dysmorphic embryo (CRL=11.1 mm) and the gestational sac (18 mm x 19 mm) at 14 days from the procedure. Serum B-hCG test was performed at day seven of the patient’s hospitalization and scheduled every seven days for the first 4 weeks after her discharge (Table 1). The most significant decrements of serum B-hCG occurred between days 7 and 14 and between weeks 4 and 6, respectively. After 12 weeks, B-hCG levels had returned to around zero (Table 1). A menstrual period occurred 87 days after the intervention. Sonographic follow up at one year from the procedure showed a normal uterine cavity with recovered trilaminar endometrium of 10.3 mm and the presence of the scar from the two previous cesarean sections (Figure 3).
Discussion
We reported the case of a pregnant woman (amenorrhea age 6 weeks + 5 days) affected by CSP who accessed our obstetric department with moderate pelvic pain. The patient had a history of cesarean sections (considered the most important risk factor for CSP). Given the patient’s age and her fertility-sparing desire, we proposed a combined conservative management strategy consisting of bilateral UAE with MTX, which was successfully performed. To reduce the chemoembolization pain, an analgesic protocol was administered intra and postoperatively. A long-term follow-up regime was applied in order to monitor B-hCG levels and evolution of ultrasound images. Today, UAE is accepted worldwide as a conservative approach to treat CSP; originally, however, it was proposed to solve other obstetric and gynecological conditions such as uterine myomas, postpartum hemorrhage, abnormal placenta implantation and cervical pregnancies [13,14]. UAE has recently been suggested as a safe and effective treatment for CSP due to the effect that the embolization itself produces: it minimizes the blood flow in the uterine arteries and thus reduces the pregnancy vascularization and determines trophoblastic degeneration [15]. Moreover, the literature shows satisfactory results when UAE is used in combination with intra-arterial MTX infusion; in line with this, Xiaolin et al. concluded that it constitutes a safe and effective method to treat cervical pregnancy [16]. Similarly, for CSP, the rationale of combining MTX trans-catheter arterial infusion with bilateral artery embolization is based on enhancement of the targeted action of MTX on the embryo. Indeed, the embryo is directly exposed to a more effective MTX dose; this also results in less systemic adverse effects for the patient. Moreover, the injection of embolic material by trans-catheter access into uterine arteries makes it possible to target the tissue ischemia. Recent studies comparing MTX trans-catheter arterial chemoembolization with MTX systemic treatment highlighted that the first treatment is more effective for CSP termination, and is associated with low-level complications. In particular, it reduces the volume of bleeding as well as the length of hospital stay and allows preservation of fertility [17-19]. Furthermore, Peng et al. noted that B-hCG levels of patients treated with UAE and MTX intra-arterial infusion recovered completely to normal more quickly than women who underwent the same procedure but with systemic administration of MTX [20]. The strength of our report is the proposal of a strict follow-up model suitable for cases similar to the one presented. Blood tests were scheduled weekly for the first four post-procedure controls, and thereafter at longer intervals until normalization of the serum B-hCG levels. Our follow-up regimen allowed us to observe the absence of fetal cardiac activity, reduction of the embryo and gestational sac dimensions, and a rapid decrease of serum B-hCG levels (reductions of more than 20% at each measurement) (Table 1). Moreover, restoration of menstrual bleeding with subsequent normal endometrial pattern (Figure 3) was documented.
However, limitations related to the nature of the study cannot be excluded. In conclusion, comparing the presented study to the existent evidence-based medicine [21], our case confirms that bilateral UAE with MTX intra-arterial infusion is an effective and safe method to treat CSP, preserving the patient’s fertility. Furthermore, it makes it possible to avoid severe complications such as massive bleeding, uterine rupture and hysterectomy. Larger prospective studies and randomized controlled trials are warranted to confirm our conclusions.
Authors' contribution
FDG is responsible for the data analysis and manuscript draft. EZ, LMDG, GD and VLP contributed to the collection of data. FAG, GB and MP revised the manuscript and gave the approval.
Acknowledgements
We would like to express our gratitude to the nurses of our emergency obstetrics department for the help received during the data collection.
Conflict of Interest
The authors declare no conflicts of interest in order of this paper