Introduction
Polycystic ovary syndrome (PCOS) is a chronic and multisystemic metabolic-endocrine disorder that affects reproductive health and increases the risk of female neoplasms. Classically, PCOS is characterized by clinical and/or biochemical hyperandrogenism and anovulation [1]. Its estimated prevalence is highly variable and ranges between 4.5 and 11.2% in adult women, based on different series [1].
According to the Rotterdam criteria for the diagnosis of PCOS (2003), at least two out of three main features must be present: hyperandrogenism, persistent anovulation and polycystic ovaries with defined ultrasonographic characteristics [2]. These criteria are still valid and frequently used for the differential diagnosis of clinical conditions other than PCOS. The need for fresh consensus is recognized, since the current criteria do not consider insulin resistance or hyperinsulinemia, which are present in a significant percentage of these women. In addition, new ultrasonography equipment allows better characterization of ovarian morphology [3] and most of the ovaries considered “polycystic” on ultrasonography studies do not correspond to PCOS [4].
Although the exact etiology of PCOS is unknown, this syndrome has been associated with significant long-term health consequences, including type 2 diabetes mellitus and endometrial carcinoma [5]. Its association with obesity and a higher cardiovascular risk is also highlighted [4]; however, these factors are not part of the classical diagnostic criteria for PCOS. Regardless of body mass index and fat mass proportion, PCOS is associated with insulin resistance and hyperinsulinemia [4], as well as with a higher risk of glycemic profile alterations when compared with findings in control women with similar body weight [4]. The prevalence of early glucose intolerance among PCOS patients can reach 40% [4].
The different insulin-sensitizing drugs, improving the response to endogenous gonadotrophins, optimizing menstrual cyclicity and decreasing hyperandrogenemia, represent a potential treatment to reverse anovulation secondary to the hyperinsulinemia that is present in PCOS [6].
The present manuscript focuses on this latter concept, aiming to promote a better understanding of the complex pathogenesis of PCOS.
Physiological effects of myo-inositol
Myo-inositol (MYO) is an inositol isoform and part of the vitamin B complex [6]. MYO plays a prominent role in cytogenesis, cell growth and the synthesis and structure of cell membranes [7]. According to preclinical data, MYO acts as a precursor to the synthesis of phosphoinositides, which are involved in the regulation of various functions, including cell proliferation [7]. Circulating free inositol is captured in most tissues by a sodium-dependent co-transporter, located in cell membranes. The importance of the inositol-phospholipids-calcium system as a second messenger in reproductive function is well known [7]. Inositol participates in oocyte maturation through calcium-mediated signaling pathways [8]. It has been shown that ovarian follicles with higher MYO levels are associated with better quality oocytes and better follicle maturation, that are linked to phosphatidylinositol cycle activation [7].
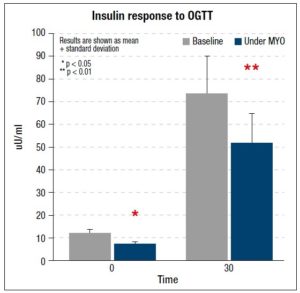
Oral administration of MYO, by optimizing insulin sensitivity (figure 1), has been shown to bring about a decrease in androgen levels, a better ovulatory response and a reduction in the level of total cholesterol [7]. Part of the effects of MYO may be mediated by increased intracellular availability of D-chiro-inositol (DCI) [7]. In addition, some actions of insulin may require the participation of mediators (such as inositol-phosphoglycans) that might also play a role as second messengers [9].
The role of MYO in PCOS
In early studies in diabetic women, it was observed that alterations in the insulin pathway could be a consequence of abnormalities in the regulation of the inositol phosphoglycans in their role as second messengers [10,11]. These first data suggested that alterations in inositol phosphoglycan metabolism act as intermediaries for insulin resistance in PCOS patients [12].
Oral supplementation with DCI, when compared with placebo, optimized ovulatory function and reduced hyperandrogenemia, hypertrygliceridemia and blood pressure in PCOS women [12].
The physiological role of inositol derivatives in gametogenesis and human reproduction has been described both in basic investigations and in clinical trials, and this has prompted further investigation of their possible application in assisted reproduction techniques [13]. As noted before, PCOS pathogenesis has been linked to an increase in insulin resistance and hyperinsulinism, resulting in different alterations in inositol signaling pathways that alter ovarian steroidogenesis. Both derivatives of inositol are insulin-sensitizing drugs, and in different studies it has been observed that MYO has effects comparable to those of DCI in terms of improving oocyte quality and regularizing menstrual cycles of women with PCOS, which in turn may have positive effects in reproductive terms via different mechanisms, including improving insulin sensitivity, increasing ovulation or reducing oxidative stress in follicular fluid [13,14].
In a randomized study involving PCOS patients, the association of MYO 2 g and folic acid 200 µg, administered twice a day for 3 months, led to significant increases in the counts of follicles larger than 15 mm, which were identifiable by transvaginal ultrasound, and of oocytes recovered during collection, compared with the use of folic acid alone and placebo [15]. In the same trial, a significant reduction in the mean count of immature oocytes related to the MYO treatment was also reported [15].
Another randomized study showed similar results in terms of improvement of ovarian function, when comparing placebo against the administration of 400 µg/day of folic acid together with 4 g/day of MYO in 92 women with PCOS [16]. Frequency of ovulation and time to first ovulation improved significantly in the MYO group, with a better and early effect on follicular maturation, which coincided with the increase in circulating levels of estradiol observed in the first week of treatment. The authors also reported positive metabolic changes in patients treated with MYO, including a significant increase in high-density lipoproteins and significant weight loss; however, no changes were reported in fasting blood glucose or insulin response to an oral glucose tolerance test [16].
It has been shown that concomitant treatment with MYO and combined oral contraceptives (COCs) improves the clinical response and the endocrine and metabolic parameters of women with PCOS. A study that included 155 users of a combined COC (30 µg of ethinyl estradiol and 75 µg of gestoden) in association with 4 g/day of MYO for one year showed that the combination significantly improved fasting insulin and glycemia, reducing low-density lipoproteins and cholesterol, and improving the parameters of the homeostatic model used to assess insulin resistance [17]. In addition, in PCOS women treated with assisted fertilization techniques, administration of MYO before hormonal stimulation increases both oocytes and embryo quality [13,15,18], and the clinical pregnancy rate [19].
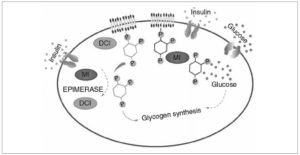
While DCI is an important molecule for glycogen synthesis, MYO is linked to an increase in glucose cellular uptake [20]. This observation, made in vivo in animal models, supports the different values of the MYO/DCI ratio observed in specific tissues, suggesting that each isomer has a different function [13]. It has been seen that organs with a high rate of glucose consumption (heart, brain) need high levels of DCI, unlike the ovaries, which do not require high concentrations of DCI for proper functioning [13].
In turn, follicular fluid of women with PCOS is characterized by a marked reduction in the concentration of MYO, in association with higher insulin resistance [21], suggesting a local depletion of MYO that may be responsible for the interference with proper maturation of the oocytes. It has been hypothesized that part of the beneficial effects of MYO on oocyte quality is related not only to its role in cellular glucose uptake that optimizes ovarian energy needs, but also to its participation in the response to follicle stimulating hormone-mediated signaling, along with induction of calcium release. This mechanism allows an adequate maturation of germ cells [13].
Data from Genazzani and colleagues strongly support the hypothesis that diabetic patients and PCOS patients with familial diabetes have a genetic predisposition to lower expression / function of epimerase. This specific endocellular enzyme, which shows decreased activity in these women, is suggested to be responsible for the compensatory hyperinsulinism shown by these patients, mainly by those with normal weight. Administration of MYO or DCI, or both together, in women with PCOS and hyperinsulinemia would be essential for the maintenance of an optimal metabolic balance and its positive compensatory role in this condition [22,23].
Conclusions
Based on current knowledge, MYO supplementation improves different endocrine-metabolic alterations and some reproductive parameters associated with PCOS. According to the available evidence on this molecule derived from inositol, MYO should be considered an effective and safe treatment for obese and non-obese women with PCOS with insulin resistance and hyperinsulinemia, administered with the aim of improving oocyte quality and maturation, the reproductive prognosis, and other associated metabolic risks in this important group of patients. Non-obese PCOS women with hyperinsulinemia appear to be an attractive group of patients for possible treatment with isomers of inositol, like MYO.
Conflict of interest: none.