Introduction:
Scalp hair cortisol concentration (HCC) has emerged as a non-invasive marker of long-term cortisol exposure. HCC can reflect perturbations of the hypothalamic-pituitary-adrenal (HPA) axis over time, such as in Cushing's syndrome or in psychological stress and depressive state [1,2].
Normal aging is associated with significant alterations in HPA activity. Previous studies that assessed the correlation between age and HCC indicated inconsistent results: some found no correlation [3–9], others observed a positive correlation [10–12], and one recent study even revealed a negative correlation [ 13].
The reason for these discrepancies is not clear, and one can argue that they might be explained by the different age and gender groups studied or by other, different sample characteristics.
Given the need to address this issue among more uniform cohorts, our study aimed to determine HCC among different age groups of women with no history of HPA axis disorders and to assess HCC correlation with age.
Methods:
Participants
In total, 40 adult female volunteers were recruited for the study. Inclusion criteria were women aged 18–50 years with regular menstrual cycles, or postmenopausal women over the age of 50 years without menstrual cycles for at least 5 years, and hair longer than 3 cm at the posterior vertex region of the scalp. Exclusion criteria included: morbid obesity (body mass index (BMI) > 35), oligomenorrhea, a history of polycystic ovary syndrome, unexplained infertility, pregnancy, lactation, active menopausal symptoms, a history of pituitary or adrenal disease, a history of breast or endometrial cancer, a history of psychiatric illness and antidepressant or antipsychotic medication use, a recent history of smoking or substance abuse (six months before recruitment), and reported use of oral contraceptives, progesterone-releasing intrauterine contraceptive device, hormone replacement therapy, or use of any glucocorticoid-containing medications during the year before recruitment.
All participants provided written informed consent and the study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee.
Clinical measures and objectives
Information about sociodemographic variables, BMI, medical history and hair-coloration status were obtained.
The primary objective was to assess the correlation between HCC and age, after adjusting for BMI, among all participants. The secondary objective was to compare clinical and laboratory parameters between pre- and postmenopausal groups.
Sample collection and preparation
In accordance with guidelines published by the Society of Hair Testing [14], hair samples of approximately 100-150 strands, 2-3 cm, long were collected using scissors from the posterior vertex, as close to the scalp as possible. Hair was ground into powder using a mini-beadbeater machine. Hair powder was incubated overnight in 1 ml of 50° C methanol, during which time it was continuously and gently shaken. Then, methanol was removed from the hair through evaporation by nitrogen stream and the residue was reconstituted in 250 μl PBS buffer (pH 8.0). Following extraction, cortisol concentrations were determined using a commercially available immunoassay with chemiluminescence detection (IBL International, Hamburg, Germany). The inter-assay and intra-assay coefficients of variation were below 9.3 and 7.3%, respectively. The final HCC was calculated as the ratio of picograms of cortisol per milligram of hair.
Statistical analyses
Descriptive statistical techniques were used to analyze the data. Data are presented as mean ± SD. The hair cortisol data were not normally distributed and hence all inferential analyses were computed using log-transformed data. Statistical comparisons between pre- and postmenopausal groups were conducted by Mann-Whitney or Chi-squared tests as appropriate. The non-parametric Spearman’s rank correlation was used to analyze the relationships between HCC, age and BMI among all participants. Partial Spearman correlation coefficients between HCC and age were calculated after adjusting for BMI. All tests were two-tailed. A p value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 21.0 for Windows (IBM Corp., Armonk, NY, USA).
Results:
Data of 39 participants were analyzed. One participant was excluded owing to incomplete sample collection. The mean age of the participants was 43.9 (±16.8) years (range 22-80 years). HCC values ranged from 1.09 to 39.82 pg/mg, with a mean value of 7.6 (±7.8) pg/mg. The mean BMI was 24.5 (±4.1) kg/m2.
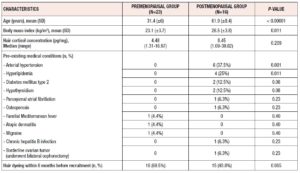
A comparison of clinical and laboratory characteristics between pre- and postmenopausal participants is provided in table 1. Mean age and mean BMI were significantly higher in postmenopausal women than in premenopausal women (61.9 (± 8.4) years vs 31.4 (± 6) years and 26.5 (± 3.8) kg/m2 vs 23.1 (± 3.7) kg/m2`, p < 0.00001 and 0.011, respectively). Median HCC values of postmenopausal and premenopausal women were 8.45 pg/mg and 4.48 pg/mg, respectively (p = 0.209). Postmenopausal women were more likely to have more comorbidities, as shown in table 1.
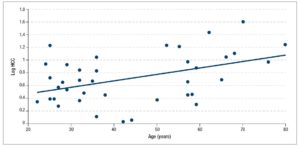
Among all the participants, HCC was positively correlated with age (Spearman's rho = 0.358, p = 0.025) (Figure 1); BMI was also positively correlated with age (Spearman's rho = 0.523, p = 0.001). HCC was found to correlate independently with age; the partial correlation coefficient between HCC and age, after adjustment for BMI, was 0.402 (p = 0.012).
Discussion:
In this study, we recruited 39 consecutive female volunteers aged 22 years and older, who were well distributed across different ages. The subjects were known to have no overt disturbances in the HPA axis. HCC was found to be positively correlated with participants' age and BMI.
Our results are in line with the findings of recent studies conducted in large, age-heterogeneous samples, which demonstrated positive associations between age and HCC [11,12]. Feller et al. examined HCC in adults aged between 47 and 82 years, and after controlling for other factors they demonstrated age as an independent predictor of HCC [12]. Moreover, Dettenborn et al. found a quadratic U-shaped relationship been age and HCC, in a sample of 360 participants aged between 1 and 91 years [10].
Still, the body of literature evidence shows contradictory results. Our results are at variance with several previous studies that did not show significant correlations between age and HCC, however, this might be explained by relatively narrow age ranges or small sample sizes of some of these studies [3–9]. Garcia-Leon et al. studied a sample of healthy Spaniards and showed a decrease in HCC with age; however, most participants were younger than 50 years of age [13].
Our findings are supported by data derived from previous studies performed on serum and salivary cortisol that demonstrated cortisol levels that increased with age [15,16]. In both men and women, mean plasma cortisol levels increased by 20-50% between 20 and 80 years of age, however, this age-related elevation of cortisol was more pronounced in women than in men [17]. Moreover, salivary cortisol, a reasonable proxy for free cortisol, showed a tendency to increase with age in both sexes [16].
Nonetheless, urinary excretion of free cortisol did not correlate with age in one study [18,19], and no difference in 24-hour urinary free cortisol (24h-UFC) levels was found between older and younger healthy women in another study [19]. While HCC and integrated 24h-UFC are both considered proxy measures of integrated cortisol levels over time, the most extensive studies have not found significant correlation between these two markers [20,21]. On the other hand, HCC was significantly correlated with integrated salivary cortisol concentrations measured repeatedly over the corresponding 1-month period [21].
Our results could be explained by several findings. As previously demonstrated, HPA system activity shows an age-related increase that might be attributed to increased basal activity and flattened diurnal amplitude of the HPA system in the elderly. In addition, it may be caused by an aging-related reduced responsiveness of the HPA axis to glucocorticoid feedback inhibition [22,23]. In our study, the median HCC of postmenopausal women was numerically higher than that of premenopausal women, but the difference between medians was not significant. However, it is plausible that endogenous cortisol production might differ according to menopausal status. Previous findings suggest that adrenal cortisol production in women may be stimulated by highly elevated postmenopausal levels of luteinizing hormone (LH) [24]. In postmenopausal women, serum LH levels have been shown to correlate positively with UFC, thus LH stimulation may induce subtle changes in adrenal function towards cortisol secretion [25]. Moreover, hot flashes are associated with higher HCC [26], although our study excluded women with active menopausal symptoms.
The present study is subject to some limitations. The sample is relatively small, and our results need to be justified in larger studies. We could not exclude other factors that may have contributed to chronic hyperactivation of the HPA axis, such as diabetes mellitus, hypertension or factors such as "subclinical" depression or anxiety. Another important variable of interest is hair-coloration status. Conflicting reports exist regarding the effect of chemical treatment of hair with dyes. Previous studies applying an enzyme-linked immunosorbent assay (ELISA) method found that hair dyeing significantly decreased HCC relative to controls [27,28], but studies applying mass spectrometry found non-significant differences [29]. Similarly, a recent study that utilized automated chemiluminescent immunoassay (the same analytical method used in our study) found no significant differences in HCC between subjects with and without dye [30]. Nonetheless, hair dyeing status seemed to have no notable effect on our results, given the fact that our study sample was homogeneous with regard to hair coloration.
Scalp hair analysis is a promising tool for the assessment of hormone secretion over extended periods of time. Besides cortisol and cortisone, several hormones have been measured in scalp hair; these include testosterone, androstenedione, DHEAS and 17-hydroxyprogesterone [31]. Long‐term steroid profiling in human scalp hair merits further evaluation of its clinical applicability in a variety of endocrine diseases, such as congenital adrenal hyperplasia, female hyperandrogenism and male hypogonadism.
To conclude, in the present study we demonstrated a positive and significant correlation between HCC and age among women. As the HCC methodology is still in its early stages, future, larger studies should be pursued in order to establish age- and gender-specific reference ranges for HCC. This may improve the clinical applicability of HCC as a retrospective marker of HPA axis function over time.
Acknowledgments
All authors contributed significantly to the writing, editing, and reviewing process. AN is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the accuracy of the data. All authors read and approved the final manuscript.
All authors declare that they have no conflict of interest.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki and its later amendments.
Informed consent was obtained from all individual participants included in the study.