Introduction
Cohort studies estimated that premature ovarian insufficiency (POI), diagnosed before 40 years of age, occurs in around 1% of women, whereas women with early menopause (EM), which occurs before the age of 45 years, is found in around 5% [1-3]. A recent meta-analysis of worldwide data reported a pooled prevalence of 3.7% for POI and of 12.2% for EM [4]. Both conditions are complex and heterogeneous in terms of diagnosis and treatment. Early loss of ovarian function may be spontaneous or iatrogenic. Induced POI/EM may be secondary to chemotherapy, radiotherapy or bilateral oophorectomy, for malignant or benign conditions, whereas spontaneous or natural POI/EM may result from several causes, including genetic, autoimmune, infectious and metabolic ones [5,6]. However, up to 60-90% of cases have an idiopathic origin, with no definite identifiable cause [7]. Different etiologies and clinical settings may influence the presentation in terms of symptoms, long-term consequences and emotional impact; management, therefore, should be multidimensional and individualized [5].
Although women with POI/EM may show intermittent ovarian activity [8], they live a significant proportion of their life in a state of profound hypoestrogenism, responsible for short-term symptoms, including hot flashes, night sweats, poor sleep quality and concentration, mood swings, vaginal dryness and dyspareunia [9]. Even long-term complications, such as a higher risk of cardiovascular diseases, osteoporosis and fragility fractures, neuro-cognitive and mood disorders [10], and increased overall mortality, have been documented because of the early hormonal deprivation [11]. Recent data from an Australian prospective cohort study reported that women with premature menopause had twice the odds of experiencing multimorbidity by age 60, and 3 times the odds of developing multimorbidity in their 60s as compared with women who experienced menopause at age 50-51 years [12]. Thus, in these women, menopause is a real endocrine deficiency and hormone therapy (HT), apart from relieving symptoms and improving quality of life, has preventive significance [13]. According to recent guidelines and recommendations from international scientific societies [14-16], in the absence of contraindications, systemic HT should be started early after diagnosis in every woman with POI/EM, and continued at least until the mean age of natural menopause, mostly around 51 years. However, there are deficits in clinical management and many women with POI/EM still receive sub-optimal treatment, with delayed HT initiation and early discontinuation, or even no treatment at all [17-20]. Also, unjustified fears of hormones, beliefs that HT is associated with breast cancer risk, and poor awareness of the benefits on cardiovascular, bone and neuro-cognitive health have been reported as main reasons for inadequate management [21].
The aim of the present retrospective observational study was to take a snapshot of hormonal management in an Italian real-life setting specialized in POI and EM. We focused on natural conditions, collecting information on etiologies and medical history, in order to gain a better understanding of the challenges to providing effective treatments.
Materials and methods
At the UOSD (Departmental Unit) of Reproductive Medicine of the University of Pavia – IRCCS San Matteo Hospital, we run a dedicated POI/EM clinic for the diagnosis and treatment of women referred to our research center from many sources (GPs, Ob/GYNs) in our area.
Starting at the beginning of 2019, we have created a database of women with POI/EM; thus far we have collected information on etiology, presentation and treatment from 181 patients. Most of the women attending our unit already have a diagnosis, formulated in the past, but request re-evaluation. In general, they come to our specialized unit to gain a better understanding of the short- or long-term symptoms associated with the condition, because of their fears and concerns about HT (duration of use, cancer risk, etc.), and in order to obtain a variety of information (on fertility issues, the aging process, etc.).
We considered only women who had signed an informed consent document agreeing to storage of their data for research purposes. The inclusion criteria were: 1) at least 1 year of amenorrhea with elevated follicle-stimulating hormone (FSH) levels, or oligomenorrhea with elevated FSH on at least two occasions in women who received their first diagnosis before the age of 45 years; 2) a history of spontaneous menarche; 3) absence of any type of pelvic surgery; 4) a complete family and personal history, including availability of previous blood tests, examinations, and any other relevant medical records; 5) the ability to report an accurate drug history regarding past or current use of HT (systemic or local) or other substances for possible relief of menopausal symptoms.
Our institutional review board approved the retrospective observational nature of the study. In the present analysis, we considered the following variables: 1) clinical characteristics; 2) etiology; 3) age at diagnosis; 4) reproductive history; 5) use of hormones and other drugs, including supplements. Other information regarding short-term symptoms and long-term comorbidities was collected, but these data are still under evaluation in a prospective design.
Statistical analysis was performed using parametric and non-parametric tests, as appropriate. Frequency tables were used to calculate data reported in the tables and figures.
Results
In our series of patients, we observed 148 women with natural POI/EM who had received a diagnosis before 45 years of age. They had a mean age of 42.9±9.8 years (range: 16-65) and their median age at diagnosis was 39 years [95%CI 37-39.4], in a range of between 13 and 44 years. Their median body mass index (BMI) was 22.4 kg/m2 [95%CI 21.7-23.2; range: 16.8-35.4]. High-level education (≥13 years) was present in 73.7% (n=109). 75.7% (n=112) of our study sample was in a stable relationship with a partner.
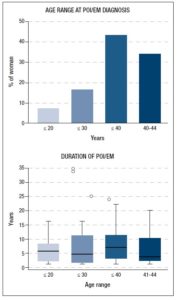
Spontaneous menarche occurred at a median age of 12 years [95%CI 12-13; range 9-18]. The median number of years the women had been in menopause was 5.5 [95%CI 4-7] with a range of between 1 and 35 years. Figure 1 (top) shows the percentage of patients distributed according to age range at first diagnosis. Women with POI (≤ 40 years) accounted for 66.2% (n=98) of the sample, whereas women with EM (41-44 years) made up 33.8% (n=50). As shown in Figure 1 (bottom), the median number of years spent in menopause was superimposable across groups with different age ranges. Age at menarche did not differ significantly between these groups, and nor did BMI (data not shown).
At the time of our consultation, most women reported characteristics of early post-menopause (1-6 years of amenorrhea) or late post-menopause (> 6 years of amenorrhea), 35.1% (n=52) and 50.7% (n=75), respectively. Only 14.2% (n=21) of these POI/EM women reported irregular menstrual cycles with characteristics of late menopausal transition (≥60 days - < 1 year of amenorrhea).
Hormone therapy and other treatment strategies
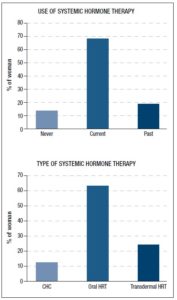
Systemic HT [both combined hormonal contraception (CHC) and hormone replacement therapy (HRT)] was ongoing in 67.6% (n=100) of our POI/EM study sample and had been discontinued by 18.9% (n=28) (Figure 2, top), while 13.5% (n=20) had never used systemic HT, due to a recent diagnosis (n=7) or evidence of vascular risks (n=13 reported previous thrombosis or uncontrolled hypertension or migraine with aura). Past users were significantly older (48.1±10.1 years) in comparison with current users (41.4±9.9 years) and never users (43.6±5.9 years) (f: 5.6; p<.005). Overall, the prescription types did not differ according to age, age at diagnosis or BMI (data not shown). Oral HRT was prescribed in 72.3% (n=81) of the cases and transdermal HRT in 27.7% (n=31), whereas CHC was prescribed in 12.5% (n=16) (Figure 2, bottom). CHC was discontinued by 8/16 women (50.0%), whereas the rate of discontinuation of HRT was lower, being 18% (n=20/112). The mean age at which women stopped systemic HT was 43.0±6.7 years, and in 28.6% of them (n=8/28) a concomitant medical condition had been diagnosed, inducing the discontinuation of the therapy, namely migraine with aura, hypertension or thromboembolic risk factors. Interestingly, the median duration of systemic HT was 48 months [95%CI 36-60.0; range: 2-420] and the last prescription had a median duration of 24 months [95%CI 12-26.7; range: 1-240]. That being so, our patients were found to have spent 67.4% [95%CI 50-84.8; range: 0-100] of their menopausal life span being treated with systemic HT (calculated upon age, age at first diagnosis and total months of use). In addition, those aged at least 51 years (n=25; 16.9%) had spent 84.2% [95%CI 46.1-100; range: 0-100] of their menopausal life span being treated with systemic HT, with a median duration of 150 months [95%CI 117-192; range: 36-420].
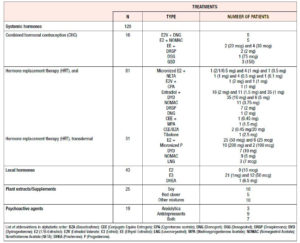
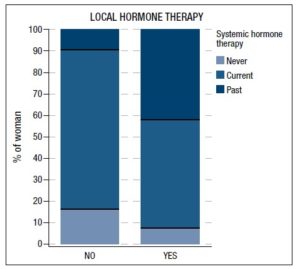
As far as local HT was concerned, about a third of our study population (29.1%; n=43/148) was currently using it, with a higher rate of prescription in past users of systemic HT (χ2:21.2; p<.0001) (Figure 3). Local HT users were significantly older (48.4±8.3 years) than non-users (40.7±9.5 years) (p<.001) and entered natural menopause a little bit later (p<.04) (40 years [95%CI 38-41; range: 22-44]) than non-users (38 years [95%CI 39-39; range: 13-44]). No differences in the rate of using local HT were found according to type of systemic HT or route of administration. Only 16.9% (n=25) of POI/EM women were currently taking plant extracts and/or other nutritional supplements for menopausal symptoms, whereas 12.8% (n=19) of them were on psychoactive agents, with no significant differences between groups. Table 1 reports the most common treatments prescribed in our series of patients with POI/EM. Of note, a wide diversity of systemic HT products was used, mainly containing standard or low doses of estrogen.
Family histories and etiologies
A positive family history of premature menopause (≤ 40 years), either in maternal (27 mothers, 5 grandmothers, 10 sisters, 2 aunts, 3 great-aunts and 1 cousin) or paternal lineages (1 grandmother, 2 aunts, 1 cousin), was reported in 30.4% (n=45), with both lineages positive in only 1 case. Some genetic abnormalities (5.4%; n=8) were documented in 3 cases with a family disposition (specifically gonadal dysgenesis, 46XX, fragile X premutation and chromosomal translocation) and in 5 cases without [3 cases of 45 X0/46XX (Turner mosaicism), 1 case of 46XX (gonadal dysgenesis plus neuro-sensorial deafness known as Perrault syndrome), and 1 case of chromosomal translocation]. Interestingly, we found that women with POI/EM presented a high rate of endocrine and non-endocrine autoimmune disorders (35.1%; n=52), with an overlap in 5 cases (Table 2).
Other relevant information
The median number of pregnancies was 1 [95%CI 0-1; range 0-7], while the proportion of women with POI/EM who had never been pregnant was 43.2% (n=64). Our study sample showed a median of 1 delivery [95%CI 1-1; range 0-4] with an abortion rate of 17.6% (n=26). As may be expected, patients never achieving the pregnant state were significantly younger at natural menopause (z: 4.7; p<.0001), with a median age at menopause of 33 years [95%CI 29-36.3; range 13-44], compared with those who reported at least 1 pregnancy (39.5 years [95%CI 39-41.5; range 26-44]). A history of use of assisted reproductive technology (ART) was reported in 17 of cases (11.5%), whereas 5 women reported a medical history of endometriosis (3.4%).
More than half of our study sample did not exercise (58.8%, n=87), whereas 25% (n=37) reported at least moderate-intensity physical activity. Smoking was reported in 22.3% (n=33), whereas 18 women were past smokers (12.2%). The median number of cigarettes per day was 10 [95%CI 7-14.8; range 1-40]. Sporadic (33.8%, n=50) or regular (3.4%, n=5) alcohol consumption was recorded in 37.2% (n=55), whereas caffeine intake was reported by 52% (n=77), with a median of 2 cups of coffee per day [95%CI 2-2; range 1-7]. The median age at diagnosis did not differ between different lifestyle groups (data not shown).
Discussion
The study population of POI/EM women described herein seems quite similar to other samples reported in the literature [6,22,23]. In idiopathic cases, familiarity, especially through the maternal lineage, strongly predicts the possibility of entering menopause earlier [24]. Autoimmunity is a frequent feature associated with POI [25] and we, too, observed several concomitant autoimmune disorders, in particular autoimmune thyroid disorders. Smoking, a well-known POI risk factor [26], was also common in our study sample.
The evidence that the majority of our patients were in late post-menopause and showed a high rate of systemic HT prescription, moreover for long treatment durations, highlighted the level of awareness of this hormone-deprived condition in Italy. On the other hand, it also highlighted the importance of having a long-term follow-up in a dedicated setting, as women with POI/EM may require specific management over time. The duration of HT is a common concern for these women and health care providers (HCPs) should reassure them that there is no specific cut-off in terms of years of use, especially when menopause occurs early. Immediately after publication of results of the Heart and Estrogen/progestin Replacement Study and the Women's Health Initiative, a significant drop in prescriptions was reported; in particular prescriptions dropped following 5 years of HT use, but age at menopause was a quite stable determinant of HT use in Italy [27]. However, our data indicated that some women discontinued systemic HT quite early (average 43 years of age), even in the absence of specific contraindications, and, in general, current users who asked for a consultation in our specialized unit were relatively young (average 41 years). Indeed, the average duration of treatment was lower than 5 years, even though it covered more than half of the menopausal life span of our sample. Of note, in the minority of POI/EM women aged at least 51 years, HT usage covered more than 80% of their menopausal life span. A recent analysis of studies reporting duration and timing of initiation of HT in POI and EM showed that women who used HT for longer than 10 years had the lowest risk of cardiovascular diseases compared with never users and women who used HT for less than 10 years, especially if they started during the perimenopause [28]. Knowledge gaps and concerns among menopausal women and HCPs are still enormous and may be a barrier to optimal management [29]. In a recent Australian survey, conducted in women with spontaneous and iatrogenic POI and EM at significant risk of fragility fractures, a low rate of HT use (27.3%) was reported and only a minority of these women (22.5%) knew about the role of HT in preventing osteoporotic fractures [30]. In a retrospective multi-disciplinary review of practice in different clinical settings in the UK, around 25% of POI women did not receive any advice regarding hormone use [31]. Our counseling revealed many fears and misconceptions already reported by other authors [20,21,32] and showed the importance of clearly explaining that controversies around HT do not apply to women younger than 50 years of age [33]. Moreover, our general impression in counseling patients was that both HCPs and women knew more about the negative impact of POI on physical and mental health, whereas EM was perceived as less harmful, a finding in line with potential under-use of HT in women between 40 and 44 years in Sweden [34].
Aware of the limited data available on this topic, the main aim of our study was to gain a better insight into the type of HT currently used in women with POI/EM. Indeed, few studies have prospectively investigated the effects of different HTs on short- and long-term variables associated with premature hormone deprivation [32], and even guidelines show some inconsistencies [35]. Very recently, Fruzzetti et al. [36] proposed an integrated and patient-based hormonal approach for women with POI that takes into account the stage of reproductive life; they further underlined the lack of evidence-based data to guide clinical practice. The choice of HT should take into account, mainly, types and doses of estrogen, contraceptive needs and future desire for fertility, and the possible need for androgen replacement [6,13,33,36]. In our study, CHC, exclusively oral, was prescribed in only a minority of women with POI/EM, namely ones who could still achieve a pregnancy because of intermittent ovarian activity [8]. The most common CHC consisted of estradiol-containing “pills”, which are considered the option with the lowest metabolic impact and with characteristics more similar to HRT [37]. In the light of the few studies showing a more favorable effect on bone protection of HRT as compared with CHC [38,39], the use of estradiol-containing “pills” seems a more sensible choice from a risk-benefit perspective, also in terms of cardiovascular health. As far as systemic HRT was concerned, the oral route was the most prescribed, probably because it is more convenient and more suitable for younger women than estradiol patches or gels, even though the latter seem to be safer in terms of long-term risks [33,36]. However, we do not really know whether these benefits apply to POI/EM women, and patient preference should therefore be taken into account to achieve long-term acceptability. The estrogen doses prescribed in our sample were standard or low and did not follow the principle of maintaining circulating estradiol between 50-100 pg/ml, a level that can be achieved with estradiol 100 mcg given transdermally or 2-4 mg given orally, according to available data in POI [40,41]. Interestingly, prescription of HRT predominantly included progestogens more similar to natural progesterone, in agreement with general guidelines [14-16]. The rate of prescription of other products, such as selective estrogen receptor modulators (SERMs), was very low, probably because these drugs are not considered suitable options for POI/EM. Indeed, we lack evidence on their use in this specific population of women. Moreover, SERMs, or even tissue selective estrogen complexes, address only some symptoms or consequences and have a fixed dose, without inducing withdrawal bleeding [42,43]. Finally, a very common HRT used in postmenopausal Italian women, namely tibolone, was rarely prescribed, even though a considerable amount of efficacy and safety evidence is available on it, including data on its androgenic properties [44], which may be relevant both in POI and EM conditions [13,14]. However, for tibolone, too, we lack published data on its use in natural EM and POI, possibly because it is a weak estrogen [44] and HCPs prefer to rely on well-established estrogen-progestogen-containing products.
Interestingly, in spite of their relatively young age, our POI/EM women displayed a high rate of use of local HTs, but these options were prescribed mainly in those who had discontinued systemic HT. Age and hormonal changes significantly contribute to vulvo-vaginal atrophy (VVA) [45] and are one of the multiple factors involved in decreased sexual function in POI women. These factors also include hypoactive sexual desire disorder (HSDD), which may be attributed to possible androgen insufficiency [46]. Other novel treatment options, specifically indicated for the management of symptoms associated with VVA, include the oral SERM ospemifene and the local dehydroepiandrosterone (DHEA) [47]. However, these were prescribed very rarely, indicating that POI/EM is perceived as a global syndrome by both HCPs and patients, and therefore suggesting that it is deemed less appropriate to treat VVA symptoms in a vacuum, even though women with POI/EM are considered at high risk of developing poor sexual function and quality of life [46].
Only a minority of women were current users of complementary therapies and the rate was similar to that reported in other series [17]. Most likely, symptomatic women with POI/EM are more motivated to initiate prescription medications rather than over-the-counter products, and even HCPs perceive the need for a standard HT [29] to treat a real hormone deficiency as opposed to a physiological hormonal decline occurring at the appropriate age. Of note, few patients in our series used psychoactive agents.
The main limitation of our study is the cross-sectional design that provides only a snapshot of attitudes and behavior in a real-life setting, without offering reliable information about patient-centered clinical decision making. However, our data confirm a general use of standard or even low doses of HT, which may be suboptimal for counteracting symptoms and conditions associated with POI/EM. Only further prospective analyses will give us information on the preventive importance of specific therapeutic choices, which seem mostly driven by the availability of certain products on the market or, possibly, by women’s preferences, rather than by tailored medical reasoning. A POI international registry, gathering information from specialized POI clinics worldwide, has been proposed and implemented to this end [48].
In conclusion, women with POI/EM deserve special care from a life-span perspective, yet there is still no clear evidence-based algorithm for their effective management. Educating both women and HCPs on the multitude of short- and long-term consequences associated with early hormonal deprivation is the key to promoting quality of life and healthy longevity in this population.
CONFLICTS OF INTEREST
During the past 2 years, Rossella E. Nappi had a financial relationship (lecturer, member of advisory boards and/or consultant) with Bayer HealthCare, Endoceutics, Exceltis, FIDIA, Gedeon Richter, MSD, Novo Nordisk, Palatin, Pfizer, Shionogi, Theramex. These companies are not involved in the present manuscript. Other authors do not declare any conflict of interest.