Introduction
Premature ovarian insufficiency (POI) is a delicate medical problem that affects young women. It consists of oligo/amenorrhea before the age of 40, characterized by elevated gonadotropins [follicle-stimulating hormone (FSH) cut-off value above 25 IU/L on two occasions more than 4 weeks apart] and low estradiol levels [1,2]. Infertility and psychological stress are common consequences of this entity, with prevalence rates of 1-3% [3]. POI is a highly heterogenous condition that may have iatrogenic, autoimmune, chromosomal, genetic or, in the majority of cases, idiopathic origin [4]. Although POI describes a spectrum of declining ovarian function and reduced fecundity due to a premature decrease in initial follicle number, an increase in follicle destruction, or poor follicular response to gonadotropins, this delicate state is not permanent and unchangeable. Intermittent or unpredictable ovarian function is present in many POI cases, and some follicles always remain (albeit a small number), as do residual egg cells capable of being recruited and fertilized.[5,6].
Hormone replacement therapy (HRT), i.e. estrogen-progestogen therapy, is a crucial treatment strategy in POI patients, specially those who are seeking fertility. An adequate and individualized hormone therapy not only treats the consequences of estrogen deficiency, but also enables recovery of ovarian function. Hormone therapy has been demonstrated to have a positive effect on folliculogenesis and subsequent conception [7,8]. The rate of spontaneous pregnancy differs in different pathological conditions, and it is generally estimated that approximately 5-15% of POI patients are able to conceive spontaneously [1,8]. Both short course and long-term estrogen administration have been found to be useful in POI patients, leading to successful pregnancy[7]. A combined hormonal regime [estrogen-progestogen therapy, dehydroepiandrosterone (DHEA), melatonin] could be better able to improve fertility outcome in POI patients [9].
Case presentation
A 21-year-old woman (weight 72 kg, height 168 cm) was referred to our department in 2004 complaining of secondary amenorrhea lasting for 3 consecutive months, accompanied by hot flushes. The patient had no previous history of oligomenorrhea. In 2002, she had had a spontaneous pregnancy and delivered of healthy male singleton weighing 3600 g.
The patient's secondary amenorrhea was evaluated comprehensively. Her serum FSH and luteinizing hormone (LH) levels were 104.2 IU/L and 61.5 IU/L, respectively; her serum estradiol level was 21.9 pg/ml, and she had a plasma anti-Mullerian hormone level of 0.08 ng/ml. These analyses confirmed ovarian insufficiency. Her prolactin level was normal (8.5 ng/ml), and thyroid function tests were within normal limits. No elevated levels of antithyroglobulin and thyroid peroxidase antibodies, or of anti-ovarian, anti-nuclear, anti-mitochondrial and anti-adrenal antibodies were found, confirming that she had no autoimmune disorders. Her karyotype was normal. Glucose tolerance test was within normal limits. Pelvic ultrasonography revealed a normal sized uterus with thin endometrium (3.5 mm) and small ovaries (median ovarian volume: 2.4 cm3) with no antral follicles.
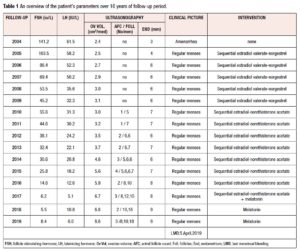
Having confirmed the diagnosis of POI, HRT was promptly started in order not only to avoid menopausal symptoms and prevent serious side effects of estrogen deficiency, but also to optimize the entire endocrine milieu, and possibly obtain resumption of ovarian activity. The patient wanted to become pregnant, but did not accept oocyte or embryo donation as a first-line treatment. Sequential HRT with estradiol valerate and norgestrel was used in the first five years (2004-2009), followed by sequential HRT with estradiol and norethisterone acetate for the subsequent 8 years. In the last year of treatment, the estrogen-progestogen therapy was combined with melatonin supplementation in a daily dose of 3 mg. The patient remained well and, reported spontaneous menstrual bleeding throughout the 13 years of HRT, until September 2017 (at which point, after failing to conceive, she had given up trying). It was the patient's own decision to stop estrogen-progestogen therapy after 13 years of HRT. She was feeling well and decided to continue only with melatonin supplementation in a 3 mg daily dose. Starting from 2004, a routine gynecological examination (including pelvic ultrasonography and endocrine testing for FSH and LH in order to estimate ovarian function) had been performed yearly in compliance with the patient's wishes. Her FSH and LH levels were seen to decline each year, in particular from 2012 (38.1 IU/L; 24.2 IU/L), while her median ovarian volume increased, in particular from 2015 (5.6 cm3), with the presence of antral follicles and increased endometrial thickness from 2010 onwards. Table 1 provides an overview of the patient's parameters during the 16 years of follow-up.
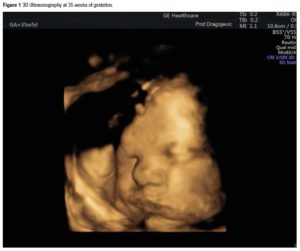
After one month of HRT withdrawal, in October 2017, the patient reported spontaneous menstrual bleeding; clinical tests showed normal FSH and LH levels (6.2 IU/L, 5.1 IU/L); transvaginal ultrasound revealed a dominant follicle in the left ovary, 15 mm in diameter, and regular endometrial thickness of 8 mm (Table 1). These clinical parameters pointed to the resumption of ovarian function. Eighteen months later, regular spontaneous menstrual bleeding failed to occur (the date of her last menstrual period was on April 5, 2019). Ultrasonography performed 2 weeks later revealed the presence of gestational sac inside the uterine cavity with visualisation of embryo tissue and of heart tube pulsation. Luteal support therapy consisted of dydrogesterone (10 mg BID orally for 60 days), in accordance with the protocol in our clinic. Serial ultrasound evaluations confirmed normal growth indices in the fetus. The fetoplacental hemodynamic indices, evaluated by means of serial color Doppler sonography, were within normal limits. Three-dimensional (3D) ultrasonography performed at the 35th week of gestation confirmed regular intrauterine growth of the fetus without malformations (Figure 1). In short, the patient's pregnancy, which reached term, was progressing normally. On December 30, 2019, a 3170 g healthy baby girl was delivered; the delivery was acomplished by cesarean section.
Discussion
POI is a delicate condition and a difficult diagnosis for young women to accept, particularly for those for whom fertility is a priority. The loss of reproductive capabilities can cause considerable distress, regardless of whether or not the patient has already had children. A carefully planned and individualized approach is required when informing patients of the diagnosis, and also when searching for the most appropriate treatment strategy.
The etiology of POI still remains an enigma in many cases. Ovarian insufficiency is heterogenous in etiology and may occur at various points in a woman's life. Initial investigation of patients presenting with secondary amenorrhea, in whom pregnancy should first be excluded, should include appropriate hormonal analyses. Although in the majority of cases the cause is idiopathic, as it was in our case, there is a need for further tests looking for a possible etiology. However, such further investigations, serving to identify the minority of women with an underlying etiology, must be targeted, in order to avoid waste of resources. Useful investigations include: autoimmune, chromosomal and genetic studies (if possible), and infectious screening [10,11]. Apart from well known iatrogenic factors, POI may be also influenced by numerous environmental factors and by many stressors. The former (radiation, chemical, toxicological influences, etc.) could be endocrine disruptors and lead to breakdown of the adaptive mechanisms in the body, while stressors may be of psychological or metabolic origin. Vujovic et al. pointed to stressors as a triggering factor in more than 50% of POI cases [12]. Indeed, the influence of excessively strong or long-lasting stressors could lead to complete endocrine milieu disruption and decreased homeostasis, in turn leading to initiation of many disorders, including POI.
POI is not necessarily permanent, but, rather associated with intermitent and unpredictable ovarian activity. Resumption of ovarian activity is possible in women with POI due to the presence of residual egg cells capable of being recruited and fertilized [6,7]. Van Kasteren and Schoemaker showed that nearly 3 out of 4 women with POI have ovarian follicles remaining in the ovary [13]. It is estimated that approximately 5-15% are able to conceive spontaneously [1,3]. Therefore, POI does not represent "loss or cessation" of ovarian activity, but a significant decrease, with possible intermittent and unpredictible activity [12]. Possible initiation of ovarian activity depends on the complete endocrine milieu, as well as on the different and individualized treatment strategies adopted. Treatment modalities that might improve reproductive outcome in patients with POI have been proposed: HRT (estrogen-progestogen therapy); immunosupression with glucocortocoids; ovarian stimulation with gonadotropins and clomiphene-citrate; dehydroepiandrosterone; melatonin [4,8,14,15].
Hormone therapy has been shown to have a positive effect on folliculogenesis and pregnancy achievement. Caroppo and D'Amato reported resumption of ovarian function after four years of estrogen-progestin treatment following an unsuccessful corticosteroid treatment [16], while Tartagni et al., showing the efficiency of hormonal treatment in improving fertility in women with POI, highlighted that FSH <15 IU/L should be achieved before commencing ovarian stimulation [17]. HRT is thought to lower gonadotropin levels into a physiologically normal range, after which the FSH receptors are up-regulated and restored [7,18]. Estrogen enhances the stimulatory effect of FSH on granulosa-cell FSH receptors as well as FSH binding to its receptors. In addition, estradiol directly sensitizes and differentiates granulosa cells, stimulates endometrial proliferation, and improves vascularization and endometrial flow; it is beneficial both for the ovary and for a well prepared endometrium, which is essential for pregnancy achievement [19]. We described our first case of a singleton pregnancy in a 33-year-old woman with presenting POI and treated with HRT. Twenty months after the start of treatment, this therapy led to maturation of one follicle and a spontaneous pregnancy ensued [7]. Gu and Xu reported a successful spontaneous pregnancy in a POI patient after 10 years of amenorrhea, intermittently treated with estrogen-progestogen therapy administered as a short course treatment [20]. An interesting case from China showed that ovarian function in tripterygium glycoside-induced POI in a woman with nephrotic syndrome was successfully restored with estrogen-progestogen and ovulation induction therapy leading to a healthy pregnancy [21].
The case here described is the first report of hormone management lasting as long as 13 years in a patient with POI of unknown origin, and resulting in restored ovarian function and successful pregnancy. An explanation for the delayed ovarian response to the treatment in our case may be hypothesized. It is presumed that chronically or highly elevated gonadotropin levels result in receptor down-regulation or desensitization. The up-regulating effect of estrogen-progestogen treatment on ovarian FSH receptors should be time-dependent and dose-dependent, therefore a prolonged course of HRT may be necessary in cases of severe ovarian receptor down-regulation. In addition, it can be hypothesized that a long-term estrogen-progestogen therapy was required to induce gonadotroph shrinkage in our patient, as well as to restore her pituitary and hypothalamus sensitivity to sex steroids. Elimination of long-term stress inductors could be an additional factor helping to restore ovarian function.
Melatonin may become a beneficial treatment for enhancing ovarian function, oocyte quality and embryo development in infertile women, particularly those who fail to get pregnant because of poor oocyte quality, and those whose reproductive life is coming to an end [15]. Melatonin treatment (3 mg daily dose) has been shown to improve fertilization rate, pregnancy rate and embryo development in infertile patients who underwent an in vitro fertilization and embryo transfer (IVF-ET) program [22]. Melatonin is a huge radical scavenger in follicles, being essential for folliculogeneis, steroidogenesis, oocyte maturation, ovulation, corpus luteum function and early embryo development. With its huge cytoprotective effect, together with its immunomodulatory one, melatonin would seem to be essential for the delaying of ovarian aging, as well for the success of pregnancy and correct fetal development. Melatonin might be a promising candidate for the treatment of POI, by reducing oxidative stress and apoptotic damage via activation of silent information regulator 1 (SIRT1/sirtuin) in a receptor-dependent manner [23]. Therefore, patients with subfertility, infertility, and even those with POI may benefit from melatonin supplementation. In our previos study, we achieved a relatively high rate (17-19%) of spontaneous pregnancy in POI patients treated with estrogen-progestogen therapy combined with DHEA and melatonin supplementation [9]. In the present case, we might hypothesize that the positive influence of melatonin in the ovarian district with its salutary effect on HRT, seen even with short duration treatment could result in the creation of an optimal hormonal milieu for resumption of ovarian function and successful pregnancy [9,15].
This case demonstrates that ovarian function in POI is a changeable condition associated with intermittent and unpredictive ovarian activity. Although it cannot be proven conclusively that it was the specific regimen of HRT given to our patient which resulted in resumption of ovarian function and pregnancy, estrogen-progestogen therapy combined with melatonin could improve fertility outcome in POI patients. Treatment of POI women must be individualized, and even a long-term hormone therapy, combined with close attention to lifestyle factors and elimination of stress inductors, could lead to complete endocrine milieu balancing with resumption of ovarian function and successful pregnancy, also restoring quality of life. Indeed, our scientific and clinical interest will be focused on further monitoring of this patient's ovarian function and reproductive potential. Finding the ideal HRT regimen for young women with POI, particularly those seeking fertility, is a real challenge and treatment should be individualized according to the choice, different needs and individual risk factors of these women. Determining the dose, duration, efficacy and safety of HRT is essential for further and larger studies. Searching for the most appropriate treatment strategy for this delicate group of young patients remains our mission.
Conflict of interest statement
The authors declare that there is no conflict of interest.