Polycystic ovary syndrome (PCOS) is the leading cause of anovulatory infertility, which shows a 72% prevalence in PCOS versus 16% in the general population [1].
Healthy lifestyle behaviors, like regular physical activity and healthy eating, should be recommended preconceptionally in all PCOS patients in order to optimize metabolic and hormonal outcomes, general health, and quality of life (QOL) [2]. This has to be accomplished before fertility treatment [3]; however, fewer than 5% of PCOS patients are satisfied with these recommendations [4] due to the fact that they are numerous and complex, and as a result adherence is poor [5]. This paper aims to help physicians better counsel PCOS patients, not only to enhance their fertility and the outcome of fertility treatments, but also to improve overall health and to decrease obstetric complications.
Methods
We performed a narrative review of the literature in PubMed (Medline). The search was restricted to publications in English, and it was performed using the following keywords: PCOS, fertility, insulin resistance, exercise, body weight, diet, inositol, melatonin, vitamin D, thyroxin, endocrine disruptors. No limits were placed on the time of publication. On the basis of an analysis of the available data, an innovative counseling approach is here proposed.
Results
Women with PCOS have reduced fertility because of the effects of obesity and metabolic, inflammatory and endocrine abnormalities, which cause reproductive dysfunction (impaired ovulatory function, oocyte quality and endometrial receptivity) and an increased risk of adverse pregnancy outcomes: gestational diabetes mellitus (GDM), gestational hypertension and associated complications [6].
Lifestyle changes should be the first line of treatment in any case of PCOS, as this approach targets the intrinsic insulin resistance (IR) in PCOS, which is the syndrome’s main pathological mechanism [3].
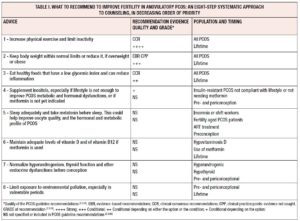
Increase physical exercise and limit inactivity
The most important lifestyle advice to give to women with PCOS is to increase their physical activity (PA). Indeed, through insulin-independent penetration of glucose molecules into active muscles, PA reduces the hyperinsulinemia caused by the intrinsic IR of PCOS [7].
After a 20-week exercise period, half (49%) of patients reported ovulation and menstrual cycle improvements [8]; furthermore, vigorous PA was associated with reduced odds of IR and metabolic syndrome (MetS) in PCOS [9].
The international PCOS guideline [2] recommends that health professionals should encourage and advise the following:
- for adults aged 18 – 64 years, either a minimum of 150 min/week of moderate intensity PA or 75 min/week of vigorous intensity PA or an equivalent combination of both, including muscle strengthening activities on 2 non-consecutive days/week
- for adolescents, at least 60 minutes of moderate to vigorous intensity PA/day, including exercises that strengthen muscle and bone at least 3 times a week
- PA to be performed in at least 10-minute bouts or around 1000 steps, with the aim of achieving, daily, at least 30 minutes on most days
For modest weight loss, prevention of weight regain and greater health benefits in PCOS:
- either a minimum of 250 min/week of moderate intensity PA or 150 min/week of vigorous intensity PA or an equivalent combination of both, and muscle strengthening activities involving major muscle groups on 2 non-consecutive days/ week
- sedentary, screen or sitting time should be minimized as it is an independent risk factor in PCOS [10]
As adherence to these recommendations is the main problem, it is necessary to explain the immediate emotional benefit of exercise. Exercise can significantly reduce body image distress, despite no change in BMI [11], and depression (HR 0.61; 95% CI: 0.88-0.33), which is more prevalent in PCOS patients [12].
In conclusion, according to PCOS guidelines [2] and literature, the first piece of advice to give any PCOS patient is to increase physical exercise and limit inactivity, and to be active even during pregnancy in order to reduce these patients increased obstetric risk, as well as long-term consequences of the intrinsic IR of PCOS (see table I).
Keep body weight within normal limits or reduce it if overweight or obese
Weight gain among women with PCOS is significantly greater than among the general population: mean difference 2.6 kg over 10 years (95% CI 1.2-4.0) [13]. Any unit of BMI increase is associated with a 9% higher prevalence of PCOS [14].
Abdominal adiposity, or central obesity (waist circumference >80 cm in Europe), is associated with higher hyperinsulinemia and IR, which lead to stronger hormonal and metabolic abnormalities such as hirsutism, infertility, diabetes mellitus, metabolic syndrome, cardiovascular disease and female cancers [15]. It causes ovulatory dysfunction, irregular menstrual cycles, longer conception times and a poor response to ovulation induction, and it is also associated with increased miscarriage, hyperglycemia, pre-eclampsia, perinatal morbidity, fetal macrosomia, and a greater potential for the trans-generational transmission of obesity and adverse metabolic features [16]. Obesity, combined with IR, PCOS and diabetes, has more than additive adverse outcomes, and PCOS could be considered an early expression of MetS [17].
Obese individuals have increased estrogen concentrations due to overexpression of aromatase in the adipose tissue. Along with increased oxidative stress, lipotoxicity and abnormal concentrations of adipokines, this directly affects the gonads, peripheral reproductive organs and embryo. Obese individuals need increased doses of ovulation induction medication and have worse live birth rates in assisted reproductive technology (ART) treatments. Weight loss can improve reproductive outcomes and restore fertility [18].
All overweight or obese PCOS patients must be advised to reach achievable goals, such as a 5% to 10% weight loss within six months. There should be an energy deficit of approximately 30% or 500 to 750 kcal/day (1200-1500 kcal/day) and the ways to achieve the diet should be formulated and planned, also considering individual energy requirements, body weight and PA levels [2]. In addition, ongoing assessment and monitoring is important during weight loss and maintenance.
Pharmacological ovulation induction should be discouraged in women with PCOS who are morbidly obese (BMI >40 kg/m2) until weight loss has occurred [19]. Bariatric surgery is known to improve the markers of PCOS, influencing different aspects of fertility, including anovulation, hirsutism, hormonal changes, IR, sexual activity and libido. This option should be considered even for PCOS patients with BMI >35 kg/m2, after the failure of a structured lifestyle intervention [20]. The patient should be made aware of the risks of surgery and of pre- and post-operative nutritional deficiencies, and therefore pregnancy should be avoided during periods of rapid weight loss and for at least 6-12 months after bariatric surgery.
Most overweight or obese PCOS patients have already tried to lose weight and have had several negative experiences of failed attempts associated with image distress and a 3- to 8-fold increased prevalence of depression that can negatively impact the motivation needed in order to focus on self-care and improving lifestyle [21]. This is the reason we ranked body weight control as the second most important piece of advice.
Psychotherapies, like cognitive behavioral therapy and treatment of depression, should be considered in order to reduce depression, to improve self-perception and QOL dysfunction, and thus ensure better adherence to the diet [22].
In conclusion, based on evidence from literature and guidelines [2], the second most important piece of advice to give any PCOS patient is to keep body weight within normal limits or reduce it by at least 5-10% within 6 months if overweight or obese. These patients should be urged to control their weight gain during pregnancy, to limit obstetric risk, also throughout their lifetime, in order to reduce the long-term consequences of PCOS exacerbated by high fat mass (see table I).
Eat healthy foods that have a low glycemic index and can reduce inflammation
According to PCOS guidelines [2], a variety of balanced dietary approaches could be recommended, as in the general population. They state that there is no, or only limited, evidence that any specific energy-equivalent diet type is better than another in PCOS.
The Mediterranean diet (MD) is an anti-inflammatory diet that is rich in complex carbohydrates, fiber, vitamins, antioxidants and monounsaturated fat; it includes a moderate amount of animal-derived proteins. Poor adherence to the MD is associated with increases in fat mass and clinical severity of PCOS, which therefore supports the therapeutic role of the MD [23]. The anti-inflammatory effects of the MD are due to the microbiota-derived production of short-chain fatty acids, induced by the intake of dietary fiber, and the high intake of omega 3 polyunsaturated fatty acids (PUFAs) and of antioxidants contained in vegetables, fruit, extra-virgin olive oil and wine.
Low carbohydrate and low glycemic index diets have been shown to improve menstrual regularity, IR and fertility in PCOS patients in randomized controlled trials [24,25] and in a systematic review [26].
Proinflammatory foods should be limited; these include products stored for a long time, processed at high temperatures, whose texture, taste and smell have been artificially improved and/or contain high concentrations of advanced glycation end (AGE) products. AGE products increase androgens and IR and contribute to ovulatory dysfunction in PCOS [27].
In conclusion, according to guidelines [2], the third piece of advice to be given to PCOS patients is to eat healthily, following the same recommendations given to the general population. According to the literature, PCOS patients may prefer foods with a lower glycemic index and that help to reduce inflammation, like those found in the MD. They should be told to make appropriate food choices during pregnancy and throughout their lifetime as they are at higher risk of metabolic, cardiovascular and oncological diseases, even though there is still no agreement on which is the best diet for PCOS (see table I).
Supplement inositol, especially if lifestyle is not enough to improve PCOS metabolic and hormonal dysfunctions or metformin is not yet indicated
Inositols, both myo-inositol (MI) and delta-chiro inositol (DCI), are nutritional supplements which act as insulin sensitizers. MI improves ovulatory function by increasing glucose cellular uptake and acting as a second messenger of follicle-stimulating hormone. DCI reduces peripheral hyperinsulinemia and has an anti-aromatase activity [28].
According to PCOS guidelines [2], metformin could be recommended for the treatment of weight, hormonal and metabolic imbalance in the pre- and periconceptional period, mostly in insulin resistant/hyperinsulinemic situations in addition to lifestyle interventions, while inositols should currently be considered experimental, according to guidelines [2] and two Cochrane reviews [29,30], with emerging evidence on efficacy highlighting the need for further research.
In recent studies, ovulation rate and menstrual cycles appear to improve with inositols, with beneficial effects on gene expression of proliferator-activated receptor gamma, which regulates fatty acid storage and glucose metabolism. They haves lower potency and side effects than metformin, and better effects than dieting only [31-34].
A 2020 expert consensus [35] states that MI, alone or in combination with DCI in a ratio of 40:1, is a promising treatment for PCOS, with relatively few side effects. Furthermore, inositols may be effective in decreasing the risk of GDM [36].
In conclusion, in PCOS guidelines [2] and Cochrane reviews, inositols are still considered experimental, however recent literature seems promising. Therefore, pending further research, the fourth piece of advice to be given to PCOS patients is to consider inositols, especially if their lifestyle is not enough to improve PCOS metabolic and hormonal dysfunctions or metformin is not yet indicated (see table I).
Sleep adequately and take melatonin before sleeping: this could help improve oocyte quality, sleep, and the hormonal and metabolic profile of PCOS
Poor sleep, circadian clock disruption, irregular sleep/wake schedules, artificial lighting and night-shift work may contribute to metabolic and hormonal disorders that can cause menstrual and reproductive dysfunctions [37].
Reduced follicular melatonin concentrations may be responsible for anovulation and poor oocyte quality in PCOS [38]. Melatonin secretion patterns and sleep appear to be disrupted in women with PCOS [39]. Mostly if overweight or obese, hyperandrogenic, insulin resistant and aged, PCOS patients suffer more from obstructive sleep apnea, loud snoring, poor sleep efficiency, and an increase in sleep-onset latency, restless sleep, daytime sleepiness, and severe tiredness despite adequate duration of sleep [40].
Melatonin, by combining its chronobiotic effect on circadian rhythm with cytoprotective properties, may represent an innovative strategy in PCOS. Melatonin supplementation could help to improve sleep and oocyte and embryo quality, the number of mature oocytes, the production of progesterone from the corpus luteum, and fertilization and pregnancy rates in PCOS patients [41]. Melatonin and MI synergistically enhance oocyte and embryo quality and improve ART outcomes in PCOS [42].
Melatonin has an aromatase-modulating activity, reduces insulinemia, improves IR, and reduces testosterone levels, oxidation and the PCOS pro-inflammatory state [43].
Young PCOS patients have higher circadian misalignment, delayed circadian rhythm and insufficient sleep. Therefore, as the prevalence of PCOS is increasing, these factors should also be discussed with them [44].
In conclusion, according to very preliminary literature, the fifth piece of advice to be given to PCOS patients is to sleep adequately and, in some cases, to supplement melatonin in order to try to improve oocyte quality and sleep and other hormonal dysfunctions. This is more relevant if they suffer from poor sleep, are overweight or obese, insulin resistant, hyperandrogenic and aged, or undergoing treatment with ART, but further studies are necessary (see table I).
Maintain adequate levels of vitamin D and of vitamin B12 if metformin is used
Lack of vitamin D may aggravate the symptoms of PCOS, while supplementation to maintain adequate vitamin D levels (≥30 ng/ml) could help to treat ovulation dysfunction, promote follicular maturation, and improve pregnancy rates in PCOS [45-47].
Vitamin D, and calcium, may improve menstrual disturbances and metabolic factors in PCOS [48].
High doses of vitamin D (4000 IU/day) showed beneficial effects on hyperandrogenism [49].
Combined with metformin and clomiphene, vitamin D significantly increases ovulation and pregnancy rates [50].
The use of metformin may be associated with low vitamin B12 levels [2,51].
In conclusion, according to literature or guidelines [2], the sixth piece of advice for PCOS patients is to maintain adequate levels of vitamin D, and also of vitamin B12, if metformin is used, but further studies are necessary (see table I).
Normalize hyperandrogenism, thyroid function and other endocrine dysfunctions before conception
PCOS is a heredity-related multifactorial disease, influenced by many environmental factors mainly during the periconceptional period, pregnancy, childhood and adolescence [52]. An unhealthy maternal lifestyle alters fetal developmental programming [53]. Epigenetic changes contribute to PCOS transgenerational transmission [54] and are associated with an increased risk of metabolic and reproductive disorders. Increased glucocorticoid and/or androgen exposure during critical periods of embryonic development can cause epigenetic programming of PCOS [55].
Maternal PCOS may be related to PCOS in daughters with a higher risk of cardiovascular diseases, IR and type 2 diabetes mellitus [56-58].
Hyperandrogenism in PCOS correlates with high anti-Müllerian hormone levels and low birth rates with ART [59]. Thus, normalizing metabolism and hormones before conception is mandatory.
In all PCOS patients with thyroid disease, hyperprolactinemia and non-classic congenital adrenal hyperplasia (17-hydroxyprogesterone) should be excluded. PCOS triplicates the chance of autoimmune thyroid disease (AITD) (OR= 3.27, 95%CI 2.32-4.63) and thyroid-specific autoantibodies should also be checked [60]. In women with PCOS, hypovitaminosis D is also associated with AITD [61].
In conclusion, according to the literature, the seventh piece of advice for PCOS patients could be to normalize hyperandrogenism, thyroid function and other metabolic and endocrine dysfunctions, mostly before and in the periconceptional period, in order to enhance fertility and to try to limit epigenetic transmission of PCOS and other pathologies to future generations (see table I).
Limit exposure to environmental pollution, especially in vulnerable periods
Pollution and endocrine-disrupting chemicals may affect the development of PCOS [62,63].
Polychlorinated biphenyl exposure is associated with menstrual cycle abnormalities and failure of implantation in in vitro fertilization procedures [64].
The exposure of pregnant rats to chemical mixtures caused PCOS-like symptoms as far as the third generation [65].
Serum bisphenol A is associated with PCOS and it might be involved in the IR and hyperandrogenism of PCOS [66,67].
Unfortunately, many methodological problems limit research on pollution and endocrine disruptors, but patients could be told to at least reduce excessive caloric intake, in order to reduce exposure to endocrine disruptors and reduce storage of lipophilic pollutants [68]. This could also be a motivation to comply better with body weight control measures.
In conclusion, according to very preliminary literature, the eighth piece of advice for PCOS patients is to try to limit their exposure to environmental pollution, especially periconceptionally and in vulnerable periods. This is a precautionary measure, to be applied pending further evidence (see table I).
Discussion
PCOS is the most frequent cause of anovulatory infertility and lifestyle interventions could help increase the natural birth rate in affected women [70,71].
This review focuses on preconceptional counseling, as opposed to pharmacological treatment, in PCOS women wishing to conceive. Ovulation induction could be performed with anti-estrogens, like clomiphene or letrozole, and/or insulin-sensitizing agents, such as metformin [72,73].
Patients should be told that reducing visceral fat through a change of lifestyle helps in reducing excessive visceral conversion of androgens to estrogen, in the same way as letrozole does. Lifestyle recommendations reduce IR, as does metformin, thereby helping spontaneous ovulation and improving clinical pregnancy and live birth rates, also in the context of treatment with ART [74].
Preconception counseling aims to enhance spontaneous conception through lifestyle interventions and supplements, reducing both the need for ovulation induction and obstetric risk in PCOS patients [75].
The role of the microbiome and probiotics in PCOS is an emerging and promising field of research [76]. Synbiotics and probiotics in women with PCOS improve hormonal, oxidative and inflammatory indices in the condition [77], however, it is too early to draw specific therapeutic indications.
Limits to the advice given are that PCOS is a multifaceted and heterogenous disease and different phenotypes may respond differently to interventions [78]. Cochrane reviews found low quality evidence and no study showing that lifestyle directly affects live birth rates [79]; however, this may have been underestimated because of low adherence. As PCOS carries a 15-fold higher prevalence of infertility, ongoing randomized controlled trials will evaluate whether high-intensity training may improve fertility, independently of BMI [80].
The role of inositols versus metformin is an open debate and the preliminary data are promising [81].
Infertile patients could be further motivated to improve lifestyle informing them that, it not only increases spontaneous fertility, it also decreases their risk of obstetric and cardiovascular diseases and that of cancers associated with physical inactivity, adiposity, insulin resistance and infertility [82].
Conclusion
Anovulatory PCOS patients who are trying to conceive should be told that the first line of treatment is lifestyle improvement. The main components are physical exercise, reduction of sedentary behaviors, and weight normalization or maintenance of body weight within normal limits. A healthy diet, low in simple sugars and with anti-inflammatory effects, ought to be recommended. However, some supplements could help. Inositols may improve insulin sensitivity, ovulation rate and oocyte quality, if melatonin is added. Vitamin D, vitamin B12 and thyroxin could be supplemented if deficient. The metabolic and hormonal milieu should be as physiologic as possible and exposure to pollutants should be kept to a minimum, particularly in the periconceptional period. If a pharmacological treatment is necessary, the same advice could accentuate the effects of drugs and/or reduce their risks, like multiple pregnancies or ovarian hyperstimulation syndrome.
The author(s) declare(s) that there is no conflict of interest