INTRODUCTION
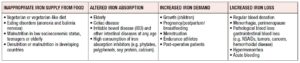
lron deficiency (ID) occurs when absorption of dietary iron is not sufficient to cover loss of iron and the body’s iron requirements. The most severe form of ID is iron deficiency anemia (IDA), which develops after depletion of physiological iron stores. IDA is the most common nutritional deficiency worldwide, occurring in 1.24 billion people, mostly women and children in developing countries [1]. The prevalence of iron deficiency without anemia (IDWA) is unknown although it has been suggested that it can be twice that of IDA [2]. Iron deficiency anemia is due to diets that are poor in bioavailable iron, and therefore inadequate to meet iron requirements. This problem is particularly important in high-risk groups like women and children [3-5]. Those at risk of ID may have one or a combination of many risk factor(s) (Table 1).
The benefit of iron supplementation in the treatment of IDA is well established and it is clear that wider use of iron supplementation in developing countries to provide bioavailable iron would have a substantial effect on relieving global ID.
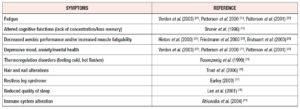
Recent studies have indicated that anemia per se is less important from a public health standpoint than the effects on health associated with tissue ID, as ID can have serious consequences (Table 2) [6-11]. In developed countries such as the USA and countries in Europe, ID is less frequent and less severe, with IDA occurring in 1 to 3% of adult men and post-menopausal women [12,13]. lron deficiency without anemia (IDWA) predominates. In this form of ID, a slightly negative iron balance reduces the body's iron stores but the hemoglobin (Hb) concentration remains normal. lron deficiency without anemia is perceived to be a less severe, milder form of ID, although there are conflicting reports concerning its symptoms.
In the last 20 years, guidelines for the management of ID have been based on the perception that mild ID, such as IDWA, may have only subtle health consequences, and on the view that preventive iron therapy in risk groups has no important clinical benefits in well-nourished populations in developed countries, and that widespread iron supplementation could even be harmful [14]. However, there is increasing evidence that ID both with and without anemia should be treated with iron supplementation. In this review the clinical consequences of depleted iron stores and the treatment of ID, particularly IDWA, are discussed.
METHODS
Information sources
In January 2020, we searched the Embase, Medline and Cochrane library databases for potentially relevant publications, considering all dates from January 2009 to January 2020. In order to maximize the number of publications meeting the selection criteria, the medical departments of companies marketing oral iron supplements were contacted in order to request publications not identified during the electronic search. Reference lists from studies initially selected and from existing reviews were also searched to identify any additional relevant studies not identified by the electronic searches.
Search strategy
The following search terms were used: “iron deficiency”, “oral iron”,” iron supplements”, “anemia”, “iron deficiency without anemia” and “iron adverse events”. No limits were set in terms of study design or dose of oral iron. The search was performed for publications in English, French or Spanish. Articles in other languages for which a translation was available in one of the 3 study languages were also included.
Study selection
Titles and abstracts of all the studies retrieved were reviewed by a single reviewer (CCB) to initially exclude clearly irrelevant publications. Five per cent of the articles excluded at this point were reviewed to confirm their irrelevance. The abstracts of the remaining articles were re-evaluated and any designated for exclusion were reviewed and confirmed by a second reviewer (LQ). The full text of each article selected was obtained and reviewed by the two reviewers to ensure the article met the inclusion criteria. Discrepancies were resolved by consensus or consultation with a third part if no agreement could be reached.
Eligibility criteria and data extraction
Publications were included if the full text of the article was available and data were clearly reported. All types of clinical studies were included. For each study included, one reviewer extracted the data and the second reviewer confirmed the selected data. Any potential discrepancies were resolved by consensus and, if necessary, by consulting a third reviewer.
Statistical analysis
A descriptive analysis of the data obtained was performed.
RESULTS
Medical consequences of IDWA
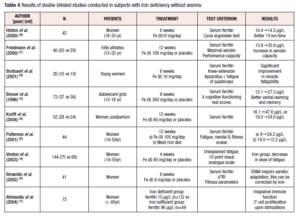
The evidence indicates that even mild ID (serum ferritin <20-35 ug/L) can result in symptoms such as fatigue, altered cognitive functions, decreased aerobic performance, restless legs syndrome and reduced quality of sleep [15-18]. Symptoms that may be related to IDWA, such as lowered physical work capacity or mental changes diagnosed by general practitioners, are not always specific (Table 3) [10,15-22,24-26]. The relationship with IDWA is therefore best analyzed according to changes in symptoms after double-blind treatments with iron and placebo over a sufficiently long period. Several intervention studies have been performed in subjects with IDWA (Table 4) [10,18-20, 22-26].
Physical capacity in subjects with IDWA
It is well known that aerobic performance capacity in athletes is reduced in IDA because of a decrease in total Hb mass, which causes insufficient oxygen transport to peripheral tissues [23]. However, there is controversy over whether IDWA limits performance in sport. Three recently published randomized, placebo-controlled studies provide evidence that performance is impaired if iron stores are depleted while Hb remains within the normal range (Table 4). In a study by Hinton et al. [24] iron-depleted non-anemic women received 10 mg of elemental iron or placebo twice daily for 6 weeks. After 2 weeks of treatment, endurance training was initiated in these physically active, but untrained women. After 4 weeks of endurance training, the time to complete a 15-km time trial was found to have improved twice as much in the iron-treated women as in those receiving placebo. After 6 weeks of iron treatment, ferritin was significantly increased and soluble transferrin receptor (sTfR) significantly decreased, while these measures remained unchanged in the placebo group. Hb and maximal oxygen uptake (VO2max) did not change in either group.
In a study by Friedmann et al. [25], 40 young elite non-anemic athletes drawn from a variety of sports (23 females, 17 males) and showing low ferritin were recruited and randomly assigned to an iron-treatment or a placebo group. For 12 weeks, while the athletes continued with their usual training program, they received either elemental iron 100 mg or placebo twice a day. Ferritin levels increased significantly only in the iron-treated group, while Hb concentration and total Hb mass did not change in either group. After iron repletion, VO2max was significantly increased; furthermore, performance in a highly intensive treadmill run, which caused exhaustion within 2 to 4 minutes, was also significantly and even more impressively enhanced, due to improved aerobic capacity, in the subjects receiving iron. In an investigation by Brutsaert et al. [26], 20 iron-depleted non-anemic untrained women were treated with 10 mg of elemental iron or with placebo twice a day for 6 weeks. In the iron-supplemented group, significantly improved fatigue resistance in dynamic leg extension exercise was observed, whereas no changes in Hb or ferritin values occurred, although transferrin saturation rose significantly.
Effects of IDWA on pregnancy and lactation
There are no clear data concerning IDWA during pregnancy and lactation and its relationship with feto-maternal morbidity. However, it is known that during pregnancy, early ID (first trimester) without anemia will result in anemia beginning around the second trimester (maximal red cell expansion) if no supplements are given [27]. In other words, preconceptional ID is a major risk factor for developing anemia in pregnancy and for its numerous negative effects. Iron requirements during pregnancy are double those of the pre-pregnancy period, due not only to the red blood expansion, but also to the fetal and placental development [28]. lt is known that neonates of woman with low or empty iron stores at term have lower iron stores during neonatal growth and adolescence. Iron supplementation should be routinely indicated during pregnancy unless iron stores of about 500mg (serum ferritin >70ug/L) are present at the beginning of pregnancy (achieved by approximately 15-20% of women in Western countries) [28]. The benefits of iron supplementation postpartum are indicated in a study by Krafft et al. [29]. Lactating women with low iron stores show lower iron transfer to the milk. Furthermore, ID in early pregnancy causes increased placental angiogenesis, which results in a so-called feto-placental miss ratio, an underlying cause of diseases after birth (fetal programming) [30]. Currently, other consequences of pure ID during pregnancy and lactation on mothers' physical or mental performance are poorly investigated [31].
Fatigue and mental and somatic health and their relationship with iron deficiency
Fatigue is an unspecific symptom, responsive to iron therapy, that has been documented for more than 150 years [17,20,22, 32,33]. In a study by Verdon et al., 144 non-anemic women aged 18-55 years (mean serum ferritin 30 µg/L) received either 80 mg Fe(II)/day or placebo for four weeks [20]. The level of fatigue decreased significantly in the iron-treated group. A subgroup analysis showed that only women with ferritin <50 µg/L improved with oral supplementation. In a study conducted in Australia, 44 women with IDWA (serum ferritin <20 µg/L) were randomly allocated to either iron supplementation or a high-iron diet for 12 weeks (Table 4) [22]. Mental health and vitality scores increased in both intervention groups; ferritin increased more under iron supplementation.
The possible relationship between IDWA and mental and somatic health symptoms like anger or fatigue were studied by Sawada et al. [34] in a group of young Japanese young women (18-22 years). These authors concluded that fatigability, anger and tension were significantly higher in IDWA subjects than in normal subjects. These women also showed a higher proportion of neurotic tendencies. These findings may suggest that IDWA could be a risk factor for the development of anger or fatigue in young women [34].
In all these studies, various parameters of physical or mental health were clearly connected with IDWA. It is particularly worth noting that some of these unspecific symptoms improved after treatment with oral iron.
The immune system is related to iron status
Another critical function that could be impaired in mildly iron-deficient individuals is immune function. In a study by Ahluwalia et al. [19], 72 homebound, elderly, apparently healthy women provided blood for comprehensive evaluation of iron status and cell-mediated and innate immunity. Women were classified as iron deficient on the basis of multiple abnormal iron status test results. In iron-deficient women, T cell proliferation upon stimulation with concanavalin A and phytohemagglutinin A amounted to only 40-50% of that found in iron-sufficient women. lt was concluded that ID is associated with impairments in cell-mediated and innate immunity and may render older adults more vulnerable to infections.
Treatment options
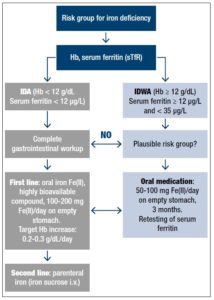
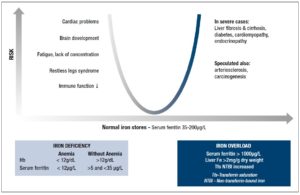
There is good evidence suggesting that every case diagnosed with ID, especially symptomatic patients, should be adequately treated with iron, even in the absence of anemia [35] (Figure 1). The treatment should always be initiated following medical consultation, and every severe case of ID should be regarded as a serious clinical symptom that requires diagnostic evaluation of the underlying disease. Because daily iron loss in adults is in the range of 1-2 mg/day, moderate to severe IDA resulting in a deficit of 1000 to 2500 mg iron is, in most cases, due to abnormal blood loss (hypermenorrhea or gastrointestinal bleeding) [14]. Therefore, in all these patients, a complete gastrointestinal evaluation including colonoscopy and gastroduodenoscopy should be obligatory.
In IDWA (Hb 12 g/dl; serum ferritin >12 µg/L), as discussed above, there are good arguments for treatment with oral iron for at least 3 months in order to replete the iron stores completely, i.e. to reach a serum ferritin value of ideally >50 µg/L. The lower cut-off value of serum ferritin for intervention with iron supplementation is less well defined. Some authors hesitated to give iron to individuals with serum ferritin <20 µg/L. Others take <35 µg/L or, in the elderly even <50 µg/L, as an indication of partly depleted iron stores which are already having physiological consequences [20].
Treatment with oral ferrous iron preparations is known to be effective and safe in most patients. Despite much progress also with parenteral iron preparations, oral iron therapy is clearly the first-line treatment in all subjects with diagnosed ID, because of safety aspects. Among oral iron preparations, the use of any ferrous salt (mostly ferrous sulfate) is generally recommended in the literature, albeit without much discussion of the relevance of the bioavailability of a given pharmaceutical iron preparation. Interestingly, this type of preparation has been studied in detail in some countries with some significant results [36-39]: i) all ferric iron preparations tested so far, even including ferric hydroxide polymaltose, a market leader in some countries, have only very limited bioavailability; and ii) there are considerable differences between ferrous preparations [38]. Differences in bioavailability are linked to the limited solubility and absorbability of iron in the duodenum and upper jejunum, the complex chemistry of iron at neutral pH, and the strong influence of the galenic composition of the respective drug. The bioavailability of any pharmaceutical iron preparation should therefore be documented and only iron preparations that are highly effective and safe should be used as iron supplements.
The efficacy and also the frequency of side effects depend upon the administered daily dose and the application modus. In adults, a typical dose of 60-100 mg/day Fe is normally sufficient to increase the Hb concentration by 0.2-0.3 g/dl/day in a non-bleeding anemic patients [27, 38- 40]. In patients with substantial blood loss (hypermenorrhea, gastrointestinal blood loss), up to 200 mg of elemental iron/day in 2-4 fractions is possible without serious side effects [41]. The best bioavailability is found when iron is taken on an empty stomach together with water. This can be stabilized by combination with fruit juice (ascorbic acid), whereas the use of tea, milk, cola, coffee or cereals should be avoided as these contain substances that act as inhibitors of iron absorption [42-44]. lf administered together with a meal, iron supplements may be better tolerated in some individuals, but at the cost of substantially decreased bioavailability [42].
Parenteral iron therapy is occasionally necessary and helpful in patients who are intolerant of or unresponsive to oral iron therapy, in patients with "functional ID" due to recombinant erythropoietin therapy, or in cancer patients [45]. One particular consideration arises in relation to its use in patients with inflammatory bowel disease, in which oral iron seems to interact with affected cells in the intestinal tract. In patients with Crohn's disease or ulcerative colitis, it has been shown that oral ferrous fumarate, but not intravenous iron sucrose, increased clinical disease activity [46].
Three parenteral iron products are available: iron dextran, ferric gluconate and iron sucrose. lron dextran administration is associated with a high incidence of adverse reactions including anaphylaxis and death [47]. The newer formulations are better tolerated, although serious side effects have been reported with ferric gluconate, whereas recommended doses of iron sucrose appear very safe with little risk of anaphylactic reactions [48-51]. These data were collected during short -term acute treatments, and although parenteral iron achieves seemingly favorable short-term results, there are no data to support its safety in the long term. However, there are reasons to suggest possible iron overload with chronic use [52].
Oral iron does induce side effects, which mainly affect the gastrointestinal tract; approximately 10-20% of treated individuals discontinue iron supplementation due to side effects. Gastrointestinal symptoms include nausea, vomiting, epigastric pain, eructation, pyrosis, meteorism, colic pain, flatulence, constipation, thin feces or diarrhea [53,54]. Whereas individuals with severe IDA may refuse to take any kind or oral iron preparation due to side effects, the majority of individuals have only mild problems [54]. A systematic review published in 2013 evaluated the tolerability of several iron supplements based on data obtained in publications. Extended-release ferrous sulfate with mucoproteose appeared to be the best tolerated of the different oral iron supplements [55].
Is there a risk from iron medication?
The widespread use of iron prophylaxis in countries with a high prevalence of severe IDA is effective, although it can also have adverse effects under certain conditions [56]. A recent community-based study in children in Zanzibar indicated that iron supplementation without restriction to iron-deficient subjects may be associated with increased risk of hospitalization and mortality due to malaria [57]. Plasmodium falciparum has been shown in in vitro studies to be less efficient in infecting iron-deficient erythrocytes than iron-replete erythrocytes, which suggests that ID may have a protective effect against malaria infection [58].
The most frequent argument against iron supplementation in individual subjects concerns the theoretical risk of chronic toxicity of iron as a reactive transition heavy metal. With regard to the treatment of athletes with iron, for example, a critical review underlined an important rule: "first do no harm" [59]. However, this argument is sometimes used without enough detailed thought and discussion.
lt is clear that any indiscriminate iron supplementation carries the risk of inducing clinically relevant iron overload in individuals homozygous (Northern European descent, prevalence, 1:200) or heterozygous (1:10) for the C282Y allele of the HFE gene [60]. However, these subjects show increased and not decreased serum iron and serum ferritin values, and can easily be distinguished from subjects with IDWA. The question of whether non-indicated, prolonged (up to years), high-dose (60-100 mg/day) oral iron treatment can or cannot induce iron overload in an individual with a normal iron regulation system is debated in the literature. In theory, the formation of excessive storage iron should stimulate hepcidin synthesis in the liver, which should then inhibit intestinal iron absorption. There are some case reports available in the literature that describe substantial liver siderosis after prolonged oral iron intake [61,62]. However, this was before HFE and the hepcidin era and the presence of a genetic hemochromatosis was not excluded. lt should also be emphasized that in this situation, a high ferritin value (>300 µg/L) would be a clear sign of misuse of iron treatment.
Another important question is to what degree is iron loading is harmful? We can learn from iron overload diseases that the degree of excessive whole-body iron storage is correlated with the severity of organ damage in the liver, pancreas and heart. The situation in the liver is studied in the most detail. In hemochromatosis and in post-transfusional siderosis, progression of hepatic fibrosis has been observed for liver iron concentrations >22.3 mg per g [62], and >16 mg/g dry weight [63], respectively. With gene testing available in hemochromatosis it became obvious that most diagnosed patients are moderately iron loaded but have mild or even no clinical symptoms [64,65], which at least indicates that moderately increased storage iron is not extremely risky in humans. Nevertheless, many publications still favor the "iron hypothesis", which suggests a fundamental role of even mildly increased iron in the development and progression of atherosclerosis or cancer [66,67]. However, it should be pointed out that neither cardiovascular nor tumor diseases (except hepatoma in cases with liver cirrhosis) are more pronounced in patients with clinical hemochromatosis and very severe iron overload [59,68]. As found in the famous Salonen publication [69] there is a correlation between high ferritin values and the risk of heart infarction, but here ferritin acts as an acute phase protein in a disease state and not as an indicator of iron overload [70,71].
In conclusion, when iron is used and carefully monitored in individuals with ID, the “iron hypothesis” should not be relevant. This is shown in Figure 2 with a u-type risk profile. The goal is to normalize the body’s iron stores in both respects. The high risk on the right side of the figure with very severe iron overload should not be a reason for neglecting the risk of ID on the left side, which should be treated with the appropriate iron formulation.
CONCLUSIONS AND PERSPECTIVES
IDWA is an under-recognised, underdiagnosed and certainly undertreated entity. The balance of current evidence strongly indicates that to ensure optimal health and development, it is important to prevent and treat even mild ID not only in growing individuals and menstruating and pregnant women, but also in endurance athletes and the elderly.
Considering the ingenious systems available that control the absorption and metabolism of iron, it is paradoxical that ID is still the most common deficiency disorder in the world and the main remaining deficiency in the developed world. A reasonable explanation may be the marked changes that have occurred in human nutrition. The current low-energy lifestyle has further led to a situation where the risk of nutrient deficiencies, especially ID, have increased. IDWA is not easy to diagnose, and any chosen cut-off value of diagnostic parameters of iron status (serum ferritin, sTfR) will have either low specificity or low sensitivity. Serum ferritin remains the diagnostic gold standard in practice. Values below 20-35 µg/L make ID the most likely diagnosis and do at the same time exclude substantial iron overload. lt is therefore very important in practice that iron supplementation is always based on actual serum ferritin values in order to prevent inappropriate doses from being administered. Bearing this in mind, all the risk profiles for inducing iron overload by iron supplementation are irrelevant. Several intervention studies show that iron supplementation in subjects with IDWA is effective and helpful for the patient. It may also prove cost-effective for the healthcare system, as further diagnostic costs are minimized. Nevertheless, parallel to iron supplementation, the cause of IDWA should be carefully explored. Faced with a patient outside a classical risk group for ID, the possibility of occult blood loss should always be related to the development of ID. lron deficiency without anemia could be a transient stage to severe ID, in which a complete gastrointestinal workup is necessary in order to find the reason. The first-line treatment of ID is oral treatment using a pharmaceutical ferrous iron preparation with documented high bioavailability. Repletion of exhausted iron stores using a 60-100 mg dose of ferrous iron (Fe(ll))/day on an empty stomach can take up to 3 months. Retesting serum ferritin (>50 µg/L) can document the therapeutic success. Treatment failure is caused by low compliance (e.g. side effects), the wrong iron compound (e.g. low bioavailability), or a suboptimal administration modus (e.g. consumed with iron-chelating food ingredients).
DISCLOSURE STATEMENT
The authors declare no conflict of interest.