INTRODUCTION
Obesity is characterized by an excessive amount of adipose tissue that leads to a chronic systemic inflammatory status. A high pre-pregnancy body mass index (BMI), currently the most widely accepted method for classifying the state of adiposity, and an excessive gestational weight gain (GWG), are independently associated with several adverse pregnancy outcomes [1], both for the mother and the newborn. Thus, maternal inflammation during pregnancy is a strong candidate for mediating effects of diverse conditions on offspring neurodevelopment, with implications for long-term health [2]. In addition, among factors affecting an individual’s risk of obesity during childhood, the main ones are the increased birthweight and maternal BMI [3,4].
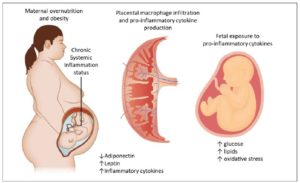
Adipocytes are mostly responsible for the increased production of several pro-inflammatory markers such as C-reactive protein, tumor necrosis factor (TNF) α, interleukin (IL) -1α, IL-1β and IL-6, IL-10 among others [5,6]. Some of them (IL-6, TNFα) are specifically produced by visceral/abdominal adipose tissue [6,7]. This metabolic activation, called “meta-inflammation”, has also been observed in pregnant women [8] (Figure 1).
While a certain degree of inflammation is essential for the implantation and maintenance of pregnancy, excessive activation is associated with several adverse outcomes [9–11]. Moreover, maternal obesity and gestational diabetes mellitus (GDM) share a similar metabolic environment, characterized by insulin resistance and a state of low-grade chronic inflammation. In addition to obesity, excessive GWG, even in lean pregnant women, is associated with up-regulation of inflammatory factors [12]. However, diet, constant physical activity (PA), and lifestyle-based interventions have the potential to limit GWG [13].
Therefore, we aimed to evaluate changes in pro- and anti-inflammatory plasma cytokines in obese/overweight women undergoing a behavioral program from the 1st trimester of pregnancy.
MATERIALS AND METHODS
Study design and protocol
This is a comparative longitudinal study, approved by the local ethics committee and registered at clinical.trial.gov as NCT02766426. Pregnant women were recruited at the Obstetrics and Gynecology Unit, Mother-Infant Department of the Policlinic Hospital - University of Modena and Reggio Emilia. All volunteers gave written informed consent prior to the beginning of the study. All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Women between the 9th and 12th week of a singleton pregnancy with a BMI ≥ 25 m/kg2, and aged ≥ 18 years were eligible.
The exclusion criteria were: chronic diseases including type 2 diabetes mellitus or hypertension, previous GDM, other medical conditions or dietary supplementations that might affect body weight or inflammatory status (such as thyroid diseases, previous bariatric surgery, smoking habits), any contraindications to practice PA. Only women who had followed the entire program, attending all the follow-up visits and therefore having a complete dataset, were included in the final analysis.
Eligible women were randomly assigned either to a behavioral program (BP) or to standard care (SC), as reported below. The randomization list was obtained by means of computer-generated random allocation in a 1:1 ratio. The numbers were sealed in numbered envelopes. After eligibility assessment, the midwife opened the envelope.
At enrollment, a detailed obstetric, family, and personal history was collected, for the assessment of exclusion criteria, by a gynecologist and a dietitian, both attendant. Moreover, weight and height were measured to assess BMI as weight (kg)/height (m)2.
The subsequent morning, in fasting conditions, a peripheral blood sample was collected for cytokine assessment. Blood sampling at the 36th week of pregnancy was also scheduled, to evaluate the same cytokine profile.
The follow-up examinations, at which GWG was measured together with anthropometric parameters, were scheduled for the 16th, 20th, 28th and 36th weeks of pregnancy, with the gynecologist and the dietitian, both attendant, evaluating the woman’s adherence to the study.
Behavioral program:
The calorie restriction intervention consisted of the prescription of a low-glycemic index (GI), low-saturated fat diet with a total intake of 1700 kcal/day [14]. The diet included a wide range of vegetables, cereals, legumes and fish, with olive oil as the main source of fat. The dietary plan consisted of three main meals and three snacks. For each meal/snack, women had several alternatives from which to choose according to their individual preferences. The diet had a target macronutrient composition of 55% carbohydrates (80% complex carbohydrates with a low GI and 20% simple carbohydrates), 20% protein (50% animal and 50% vegetable) and 25% fat (12% mono-unsaturated, 7% polyunsaturated and 6% saturated), with moderately low saturated fat levels. The daily recommended calorie intake was divided into small frequent meals to avoid ketoacidosis due to prolonged fasting [15].
A PA program was also prescribed, in which women were encouraged to spend 30 minutes/day walking, at least 4 times/week [16,17].
Standard care
Women received a general nutritional booklet regarding correct lifestyle, namely about proper food intake but without specific calorie restrictions, in accordance with the Italian guidelines [18]. These women received the standard recommendations for adequate PA.
Cytokine measurement
Immediately after blood collection, plasma was separated from erythrocytes (spin-dried at 4000 rpm) and stored at -20°C for cytokine measurement. Cytokine measurement was performed with the “Human Cytokine 2 10-Plex Array, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, TNF-α (Aushon Ciraplex ®)” at the Laboratory of the Obstetrics and Gynecology Unit, Mother-Infant Dept. of Policlinico Hospital of Modena.
Statistical analysis
Cytokine plasma levels were converted in logarithmic values, for normalization. A Chi-squared test was used for the categorical variables. For the demographic variables, we used frequencies and Student’s t-test comparisons. To compare continuous variables, the Wilcoxon rank-sum test or the Kruskal-Wallis equality of populations rank test for non-parametric measures were performed. Continuous variables are reported as median values and interquartile ranges while categorical variables are reported as numbers with % in brackets. Anova test was performed to compare the median of cytokine values at the third trimester between three groups defined according to adherence to the Institute of Medicine (IOM) recommendations; a post-hoc Bonferroni test was then performed to detect the differences between groups. We considered a p-value less than 0.05 as the threshold for statistical significance. The data were analyzed using Stata Statistics/Data Analysis software v 13.1 (StataCorp 13.1, Texas, TX, USA).
RESULTS
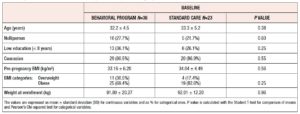
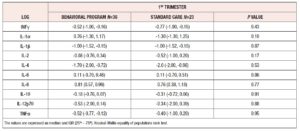
Among 70 consecutive eligible women, 59 completed the study and were included in the final analysis. Thirty-six of these women were allocated to the BP and 23 to the SC group. The reason for exclusion of women was non-attendance of follow-up appointments after enrollment. Table 1 lists the sociodemographic and clinical features of the randomized women. No differences were found between the groups. Weight and BMI categories were also equally distributed between the groups. Women reached a similar weight at 36th week (BP= 101.8 ± 18.3 kg; SC= 101.8 ± 12.8 kg, p= 0.98). GWG was also similar between the two groups (BP= 9.73 ± 6.7 kg; SC= 9.86 ± 7.6 kg, p= 0.94), as was the rate of women with GWG above the IOM recommendations (BP = 15, 41.6%; SC= 10, 43.6%; p = 0.89). Logarithmic median values of cytokines measured at baseline were similar between the two randomized groups (Table 2).
The BP group, during pregnancy, showed significant decreases in IL-1α, IL-1β, IL-2 and IL-12p70 concentrations. Conversely, IL-6 increased significantly in the same period (Table 3). The SC group showed significant decreases in IL-1β and IL-2, and, similarly to the BP group, a significant increase in IL-6. No changes in IFN-γ, TNF-α, IL-4 or IL-10 were seen in either group (Table 3).
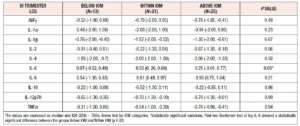
Considering that the women showing excessive GWG during pregnancy were equally distributed between the two study groups, we took all 59 patients enrolled and divided them into three categories: GWG Below, Within and Above IOM recommendations. Comparing the logarithmic median values of cytokines at the 36th week in the women divided between the three different IOM categories, no statistically significant differences were observed apart from IL-6 levels, which were significantly higher among women whose GWG was Within recommendations (Table 4).
DISCUSSION
This longitudinal comparative study demonstrates that circulating cytokines undergo significant changes throughout pregnancy, in relation to the lifestyle program followed. However, those changes seem unrelated to concomitant variation of body weight.
It is well-known that pro-inflammatory cytokines show an upregulation during gestation and parturition [8,19,20], and have been shown to be even higher in obese women compared with lean ones [21]. Conversely, literature data on the effect of lifestyle changes on cytokines in pregnancy are unclear. A large multicenter trial (the LIMIT trial) conducted in women with BMI ≥ 25 recently found no changes in IFN-γ, TNF-α, and IL-1β, 2, 4, 6, 8 and 10 between groups randomized to lifestyle intervention or standard care [22]. This lack of effect on circulating cytokines was contrary to our findings and it could be explained by the timing of intervention, which started at the 20th week, whereas we randomized our women much earlier. Walsh et al. also observed that a low-GI diet did not affect cytokines. In their study, women underwent only dietary changes, whereas we implemented constant PA in addition to calorie restriction [23].
One of the first studies that prospectively investigated the effect of sustained weight loss on circulating pro-inflammatory cytokines was conducted by Ziccardi et al. in obese women [24]. They found that circulating TNF-α, IL-6 and adhesin molecules were elevated in obese women and correlated with indices of adiposity, particularly central adiposity. Reduction of body weight resulted in a significant reduction of cytokine levels and a concomitant amelioration of endothelial function. Moreover, women with the greatest degree of visceral obesity had the greatest decrease in cytokine and adhesin levels, highlighting the role of visceral fat as a key factor predisposing to atherosclerotic disease.
It is worth noting that our behavioral program reduced several cytokines, but not IL-6, whose level was increased toward the end of pregnancy in both types of intervention. IL-6 production has been causally related to the amount of adipose tissue [5,25] , and we previously reported that at 36th week, both total and visceral fat increase in women who had undergone a lifestyle program [26].
IL-6 is a multifunctional cytokine, playing important roles in acute and chronic inflammation as well as in autoimmunity [27]. It acts as part of a cytokine network that directs the shift from innate to acquired immunity during initiation of a new immune response, through differential control of leukocyte recruitment, activation and differentiation, and apoptosis [28]. High levels of IL-6 have been detected in gestational tissues, amniotic fluid and serum during labor, suggesting that a rise in IL-6 bioavailability contributes to the normal physiological processes of parturition [29,30], together with several other proinflammatory cytokines [31].
One of the major findings of our study is the impressive decrease in IL-1α associated with lifestyle intervention. This result is particularly important because IL-1 family members are major players in the initiation and regulation of inflammation associated with obesity [32]. More specifically, novel evidence has been produced on the contribution of IL-1α to the development of obesity and insulin resistance. Plasma IL-1α levels have been found to be higher in obese than in lean mice; IL-1α inhibits adipocyte differentiation and reduces insulin signaling in murine adipocytes in vitro, while in vivo, injection of mice with IL-1α increases plasma triglyceride levels [33,34]. Moreover, oils derived from human adipose tissue activate and recruit neutrophils and macrophages through an IL-1α-dependent mechanism [35], suggesting that this cytokine may control the onset of adipose tissue inflammation [36].
We could speculate that a behavioral program prevents the abnormal increase in selected cytokines, and perhaps mitigates the negative effects of generalized chronic inflammation typical of overweight and obese women.
The main limitation of our study is certainly the small sample size due to the restrictive inclusion criteria and to the fact that we included only patients who followed the entire program and had a complete dataset. Consequently, though, a strength of our study is that the women were 100% compliant with the program in both arms, and therefore the results are not affected by any loss to follow up.
In conclusion, our findings suggest that a behavioral program started early in pregnancy in overweight and obese women leads to a selective reduction of pro-inflammatory cytokines toward the end of gestation. Therefore, it has the potential to reduce the detrimental effects of the generalized chronic inflammation status, characteristic of overweight and obese pregnant women.
DISCLOSURE STATEMENT
The authors declare that there is no conflict of interest