INTRODUCTION
Menopause is defined as the complete cessation of menstruation, for a period of at least 12 months, due to reduced production of estrogen and progesterone hormones by a woman’s ovaries [1]. Menopause can occur spontaneously (natural menopause) or be induced by surgery, chemotherapy or radiotherapy, leading to estrogen deficiency and loss of reproductive function [2].
The average age at menopause ranges between 48 and 52 years among women in developed nations, which means that the postmenopausal period constitutes approximately one third of women’s lives [3]. Genetic, environmental, racial, lifestyle and anthropometric factors influence the occurrence and intensity of menopausal symptoms [2]. The most commonly reported are vasomotor symptoms (hot flashes and night sweats), vaginal dryness, genitourinary syndrome of menopause (also known as vulvovaginal atrophy), osteoporosis, sleep disturbances, urinary incontinence, changes in libido, mood changes and fatigue [4].
Hormone replacement therapy (HRT) is the most common treatment for menopause-related symptoms, which it aims to attenuate by replacing the hormones secreted at lower levels by the ovaries in the postmenopausal, versus the premenopausal, stage [5]. HRT can be administered orally, transdermally, percutaneously, intramuscularly, intranasally, subcutaneously, or locally (vaginally), with doses and timing tailored to each patient. However, not all administration routes are easy and comfortable to apply. A study performed by Minkin and collaborators showed that postmenopausal women treated with vaginal estradiol cream, vaginal rings or vaginal tablets for their symptoms of vaginal atrophy preferred the vaginal tablets, perceiving them as “efficacious, convenient and neat to apply” [6]. Similarly, for atrophic vaginitis treatment, postmenopausal women considered an estradiol vaginal tablet more user-friendly than vaginal estrogen cream [7].
Although the oral route has been the benchmark for comparison, transdermal spray solutions containing estradiol seem to be a good and suitable strategy for the relief of menopausal symptoms [8]. In fact, transdermal administration is preferred in cases of oral treatment intolerance, alteration of liver function, hypertriglyceridemia and diabetes mellitus. This administration route bypasses the first-pass effect, providing better bioavailability and facilitating a long-term balance of estrogen levels, as well as maintenance of the physiological estradiol/estrone ratio [5]. Additionally, transdermal estradiol has little or no effect on clotting factors, lipoproteins, sex hormone-binding globulin, hepatic enzymes or C-reactive protein [9]. Furthermore, authors have reported little or no increase in thromboembolic events with transdermal estradiol compared with oral estrogens [10].
Lenzetto® (Gedeon Richter Plt., Hungary) is a transdermal spray solution containing 1.53 mg estradiol whose efficacy and safety have previously been reported [8]. Symptom attenuation and satisfaction with a therapeutic treatment seem to be determinant for women’ adherence to a treatment. Their satisfaction and acceptance of a therapeutic method can be monitored through administration of a survey [6,7,11–13]. The aim of the present observational study was to assess the level of satisfaction with Lenzetto®. Other aspects evaluated were severity of menopause-related symptoms, and ease of use and rate of continued use of this transdermal spray solution for the treatment of menopause-related symptoms by women in Portugal.
METHODS
Subjects
The subjects were healthy postmenopausal women between the ages of 45 and 65 years, inclusive. Postmenopausal was defined as natural spontaneous amenorrhea for at least 6 months or surgical menopause, with or without conservation of the uterus. In the subjects enrolled in this study, initiation of the treatment was indicated as part of their normal routine care. The subjects were physically and psychologically able to participate in the study and they were being followed up under the National Healthcare System. Participants gave free written consent to participate in the study through the informed consent form approved by the competent ethics committee of each participant institution.
Subjects were excluded if they had any known adverse reactions to the active substance or to any of the excipients. Women with known or suspected estrogen-dependent malignant tumors (e.g. endometrial cancer) were excluded from the study. Patients with untreated endometrial hyperplasia or with undiagnosed genital bleeding were also disqualified. The study also excluded subjects with a current or recent history of drug abuse or alcohol abuse, and participants with a serious illness, mental disorder or any other cause that could affect their participation.
Study design
The present study used a prospective, multicenter, observational design. The study initially recruited 237 postmenopausal women due to use the transdermal spray containing 1.53 mg estradiol for the treatment of menopause-related symptoms as part of their normal routine care. No additional diagnostic procedure was applied. Women who fulfilled all the eligibility criteria were invited by their gynecologist to participate in the study.
Participant satisfaction with the transdermal spray treatment was assessed through three self-evaluation questionnaires. A baseline questionnaire was administered after the recruitment appointment and two follow-up questionnaires were administered 3 and 6 months after they started treatment with the transdermal spray solution. The follow-up contacts were performed either by phone or email.
Measurements
The survey contained questions gathering demographic data (age, height, weight and blood pressure) and information on the clinical background of menopause. Participants were asked about: current medication; intensity of menopause symptoms before and after treatment with the transdermal spray solution; number of daily applications, zone of application and reason for any cessation of treatment; ease of use and satisfaction; lapses or failures of treatment and intention to continue using the transdermal spray solution containing 1.53 mg of estradiol.
Statistical analysis
Data collected using the questionnaires were cleaned and coded. Data were entered and analyzed using SPSS Statistics for Windows, Version 21.0 (Armonk, NY: IBM Corporation). Proportions, arithmetic means and standard deviations were used as summary statistics. Where appropriate, the Friedman test was used, followed by a post-hoc analysis with the Wilcoxon signed-rank test or the non-parametric Mann-Whitney test. Spearman correlation coefficients were calculated to assess the relationship between product acceptability or ease of use and its continued use. Correlations were based on individual level paired data. All statistical tests were two-sided and performed at the 0.05 level of significance. No adjustments were performed for the multiple comparisons carried out in the study.
As a measure of internal consistency of the implemented questionnaire, Cronbach’s alpha was calculated. For assessment of sample size, G*Power software (version 3.1.9.6) was used. Using Wilcoxon signed-rank test, α error probability of 0.05, power (1-β) of 0.9, parent distribution minimum asymptotic relative efficiency and effect size of 0.5, we calculated a sample size of 162.
RESULTS
Recruitment and follow-up
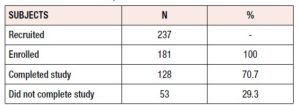
A total of 237 women were initially recruited; of these, 56 failed to meet the inclusion criteria (2 had depression symptoms, 8 did not meet the age inclusion criteria, 7 were unreachable either by phone or email, 13 had already been treated with Lenzetto®, and 26 did not start the medication). The remaining 181 women met all the inclusion criteria and 128 (70.7%) completed the study (Table 1). Among the 53 subjects who did not complete the study (29.3%), voluntary withdrawal and loss to follow-up were the main reasons identified. Response rates to the surveys were high: 173 (95.6%) women answered at baseline and 151 (83.4%) and 128 (70.7%) completed the 3- and 6-month follow-up surveys, respectively.
Values of Cronbach’s alpha for each question ranged between 0.753 and 0.795, while the value for all the items was 0.779. These values indicate a high level of internal consistency for each questionnaire (i.e. compiled at the different timepoints).
Sample demographic characteristics
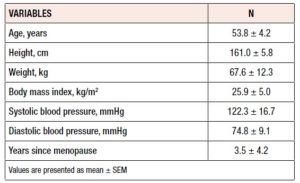
Table 2 shows the subjects’ demographic and baseline characteristics. The average age of the women was 53.8 years and approximately half were younger than 53 years. Mean height was 161.0 ± 5.8 cm, mean weight was 67.5 ± 12.3 kg, and mean body mass index was 25.9 ± 5.0 kg/m2. Systolic and diastolic blood pressure mean values were 122.3 ± 16.7 and 74.8 ± 9.1, respectively.
In 32% of women, treatment started after surgical menopause. In 32% of women with spontaneous menopause, progestins were recommended as a complementary therapy to estradiol for endometrial protection. 40% of women enrolled in this study, with natural or surgical menopause, had already taken medication to manage menopausal symptoms, however no medicine was found to be preferred. Previous treatment had lasted for an average of 3 years and had been discontinued prior to the women’s enrollment in this study.
Menopausal symptoms
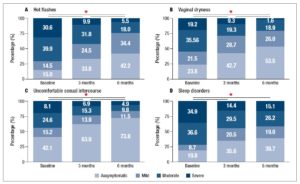
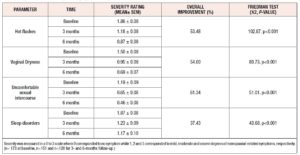
There was an overall, and statistically significant, improvement in all the evaluated menopause-related symptoms between the initial survey and both the 3- and the 6-month follow-up (Figure 1, Table 3).
Following post-hoc analysis with Wilcoxon signed-rank tests, all menopause-related symptoms showed significant reductions between the baseline and the 3 months questionnaire, between the 3 and the 6 months questionnaires, and between the baseline and the 6 months questionnaires (p<0.001); the only exception was the effect of treatment on sleep disorders between the 3 and the 6 months questionnaires, where no statistically significant difference was observed (p=0.074).
The most significant improvement concerned uncomfortable sexual intercourse whose overall symptom score increased in 61.34% (Table 3). Hot flashes and vaginal dryness scores improved in 53.48% and 54.00%, respectively.
Women preferentially applied this transdermal spray solution on the forearm. Less than 3% of participants applied on the thigh, belly, or altered between the forearm and thigh were also reported. More than 80% of women applied the transdermal spray solution one to two times per day, with approximately 50% of women applying it only once a day. The mean number of applications was 1.55±0.69, 1.61±0.72 and 1.59±0.71 at baseline, 3 and 6 months after the start of treatment, respectively. Nevertheless, during the study, the number of pulverizations did not differ over time (χ2=1.806, p=0.405).
Global satisfaction
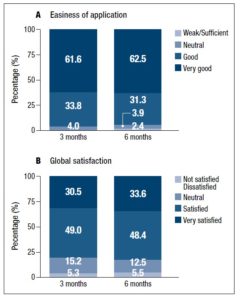
Throughout the study, more than 95% of the women reported that the transdermal spray solution was easy to apply. Women were asked to rate its user-friendliness on a 0 to 4 scale where 0 indicated “weak” and 4 indicated “very good” (Figure 2). Mean scores were 3.56±0.05, 3.54±0.05 and 3.53±0.06 at baseline, 3 and 6 months following the start of treatment, respectively. No differences were found when comparing the different timepoints. Overall, 93 to 96% of the participants considered the application of the transdermal spray solution to be easy or very easy.
Less than 5% of the women claimed to have forgotten more than 3 times a week to apply the transdermal spray solution. In fact, half of them answered that they had never missed an application, while the remaining women did not forget more than 1 to 2 times a week. Although no statistically significant correlation emerged between ease of application and missed applications, greater ease of application often led to higher adherence and compliance with the treatment.
The global satisfaction questionnaire indicated that the participants were satisfied with this transdermal system device. At month 3, 79.5% of the subjects were ‘very satisfied’ or ‘satisfied’ with the transdermal spray solution. This value increased at month 6 (82%), although the difference was not statistically significant (p=0.581) (Figure 2).
More than 75 percent of women who replied to the questionnaire expressed their intention to continue using the device. Both ease of application and level of satisfaction positively influenced treatment continuity; however, the latter had a higher impact (r=0.177, p=0.049 versus r=0.278, p=0.001).
Importantly, type of menopause – natural or surgical – did not impact overall satisfaction (p=0.277) or long-term willingness to continue using the transdermal spray solution (p=0.839).
At the end of the study, 21% of the women decided not to continue the treatment. Of these, 33% reported some discomfort or non-serious adverse events during the treatment, 38% were to change medication due to medical indication, while 14% stated that no symptom relief was achieved. The others intended to stop the transdermal spray solution application because complete symptom relief had been obtained. Notably, only one woman gave total dissatisfaction with the transdermal device as her reason for stopping.
DISCUSSION
Menopause states for the last menstrual period itself and there is frequently the need to initiate HRT. Women with menopause-related symptoms frequently seek health care and their decision to start hormone therapy is based on the severity of their symptoms. Patient acceptability and preference are very important aspects of HRT, which significantly affect patient compliance. Since long-term menopausal treatment is often required, an easy and convenient treatment should be available. The main objective of the current study was to assess satisfaction in women who received a transdermal spray containing 1.53 mg estradiol for the treatment of menopause-related symptoms.
At month 3, the participants reported significant improvements from baseline in scores for each menopause-related symptom assessed on the questionnaire, and with exception of sleep disturbance, these scores were also improved at month 6. The majority of women enrolled in this study used the transdermal spray device 1 to 2 times per day. The effects of 1.53 mg estradiol in the present study are consistent with those of the active treatment reported in a placebo-controlled trial [14]. Buster et al. also showed a significant (p<0.01) decrease in the frequency of flashes after both 4 and 12 weeks of therapy in women who had used 1–3 sprays of transdermal estradiol or placebo daily. Similarly, a multicenter open-label efficacy trial reported a significant drop (p<0.001) in all menopause-related symptom scores verified both after 3 and after 6 months of therapy [8]. Like other trials, the present study supports the use of this system for managing menopausal symptoms.
The transdermal spray device studied allows the application of incremental doses to improve effectiveness in a dose-dependent manner in women who do not achieve sufficient symptomatic control at lower doses. Moreover, the use of low-dose estrogen allows women to safely use low-dose progestin dosing for endometrial protection [9]. Nonetheless, new recommendations state that although low-dose, topical, vaginal estrogen may be given without a progestogen, any vaginal bleeding after menopause must be carefully investigated [4].
In previous studies, transdermal estradiol spray, used for the treatment of menopausal vasomotor and other related symptoms, showed the same efficacy as oral, patch and gel formulations [9,15]. Therefore, in the presence of similar effectiveness, selection criteria would be based on the pursuit of fewer adverse effects and greater patient acceptance. Higher acceptability supports patient adherence and consequently leads to safer and more effective use of medicines [16].
The women who participated in this study considered the transdermal estradiol spray to be an easy method of application, with more than 90% classifying it as user-friendly or extremely user-friendly. This high acceptability may have facilitated treatment adherence. Moreover, the women strongly approved of the transdermal spray device, with 82% stating that they were ‘satisfied’ or ‘very satisfied’ with it at the end of the study. These two variables positively affected the patients’ willingness to continue the treatment in the long term.
The findings of this study substantiate the importance of a patient-friendly local estrogen formulation for the treatment of postmenopausal symptoms. Product attributes positively impact women’s treatment choices and should assist health care professionals in the optimization of treatment.
One potential limitation of our study is that observational studies are subject to bias. Preferential referral could have occurred. Furthermore, the data were obtained through questionnaires, in which self-report discrepancies can result in under- or over-representation of symptom severity and levels of satisfaction. Also, no comparator devices were assessed directly. Nonetheless, to the best of our knowledge, this is the first study assessing women’s satisfaction with a transdermal spray device. Moreover, this study provides new insights into the relationship between satisfaction and treatment continuity. These data demonstrate that this transdermal spray device can be considered a key option for HRT to be discussed in the context of shared decision making – an option capable of ensuring patient satisfaction and adherence throughout the treatment and, therefore, of improving therapeutic outcomes.
CONCLUSION
Menopause-related symptoms improved both after 3 and after 6 months of estrogen application in the form of a transdermal spray. The vast majority of the participants reported being “satisfied” or “very satisfied” with the treatment, which confirms that this transdermal spray device is a valid option for the treatment of symptoms associated with the menopause. Thus, women who are intolerant of, or are not inclined to use, patches, gels or emulsions can benefit from the advantages of this transdermal delivery system. This study suggests that 1.53 mg estradiol transdermal spray is a valuable HRT option, demonstrating high acceptability and subject satisfaction, which enhances adherence to treatment. Accordingly, it should be considered in the context of shared decision making.
ACKNOWLEGMENTS
The authors would like to thank the medical doctors who recruited participants for the study. Data collection, statistical and editorial support were performed by an independent committee, with funding from Gedeon Richter Plc.
CONFLICT OF INTEREST STATEMENT
Daniel Pereira da Silva is a Medical Advisor of Gedeon Richter Portugal. The remaining authors declare that there is no conflict of interest.