Introduction
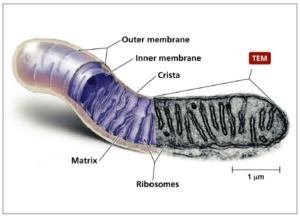
In all eukaryote cells, mitochondria are the organelles providing most of the energy used by these cells and are therefore also called the “powerhouse of the cells”. In terms of evolutionary biology, these organelles originate either from endosymbiotically living aerobic bacteria or from incorporated factual anaerobic bacteria in archaea [1]. Structurally, mitochondria have two membranes, an outer membrane and an inner membrane forming so-called cristae which are highly compartmentalized sections (Figure 1). Between these two membranes is the intermembrane space. The space enclosed by the inner membrane is called matrix. Human sperm contain about 50 to 75 mitochondria helically arranged around the axoneme in their midpiece in about 12 to 13 gyres and 2 mitochondria per gyre [2,3]. In contrast, mature human oocytes contain about 100,000 spherical mitochondria with only a small number of cristae [4].
In the cytoplasm of cells, glycolysis is an anaerobic process taking place converting glucose into pyruvate and producing four molecules of adenosine triphosphate (ATP). In the presence of oxygen, the α-keto acid pyruvate is transported by pyruvate carriers into the mitochondrial matrix and then converted by pyruvate dehydrogenase into acetyl-CoA which enters the Krebs cycle for oxidative phosphorylation (OXPHOS) [6]. Acetyl-CoA is also formed via β-oxidation from fatty acids which are transported through the inner mitochondrial membrane by carnitine/acylcarnitine [7]. The multi-enzyme complexes I-IV (complex I: NADH dehydrogenase; complex II: succinate dehydrogenase; complex III: cytochrome 3 reductase; complex IV: cytochrome 3 oxidase) located in the inner mitochondrial membrane are forming the mitochondrial electron transport chain (ETC), while complex V (ATP synthase) produces the ATP that is necessary for cellular energy needs [8].
As a result of this oxidative phosphorylation, not only ATP is produced but up to 5% of the consumed oxygen is leaving the system as superoxide (•O2-), hydrogen peroxide (H2O2) or other reactive oxygen species (ROS) compounds [9-11], which have half-life times in the milli-second (10-3 s) to nano-second (10-9 s) range [12]. These cytotoxic products accelerate cell aging and can cause numerous disorders and cell death via cell cycle dysregulation and apoptosis [13]. Therefore, with the significant increase in the atmospheric oxygen concentration about 2.3 billion years ago [14], protective mechanisms preventing extremely sensitive biomolecules developed including enzymatic and non-enzymatic antioxidant systems. Life also adapted to these higher oxygen levels by using it to produce energy in the mitochondria [15] as well as for cellular redox regulation, signaling [16] and triggering essential physiological functions such as gene regulation, cellular activities or synaptic plasticity [17-20]. Hence, H2O2 and •O2- are not only harmful, but fulfill essential physiological and cellular functions regulating functions such as immunity, proliferation, development, or steroidogenesis [21]; all depending on the concentration.
Since mitochondria developed from endosymbiotic bacteria, mitochondria have their own DNA (mtDNA) which encodes for approximately 13 protein subunits of the mitochondrial electron transfer chain, and ribosomal RNA (rRNA) and transfer RNA (tRNA) components of the mitochondrial translation system [22]. However, contrary to nuclear DNA (nDNA), mtDNA has 3 to 7 circular, much shorter, double-stranded DNA strands which are and not protected by histones and protamines like the nDNA. Furthermore, since mtDNA replicates much faster than nDNA, it does not have proofreading mechanisms and only very basic repair mechanisms, the mitochondrial genome is about 100-times more prone to mutations and mitochondrial diseases than nDNA [23,24]. In addition, since the mtDNA genome sites responsible for ROS-generation overlap with their positions are mainly attached to the mitochondrial membrane facing the matrix [25], crosslinks between mtDNA proteins can be formed increasing mitochondrial fission and mtDNA damage [26,27] eventually leading to a vicious cycle of ROS production due to damage to the ETC. In meta-phase II mouse oocytes, Lord and co-workers showed that following ovulation, increased production of ROS leads to lipid peroxidation with the formation of 4-hydroxynonenal (4HNE), malondialdehyde and acrolein [28]. In turn, these aldehydes chemically modify oocyte proteins including mitochondrial succinate dehydrogenase as primary target with subsequent loss of mitochondrial membrane potentially leading to apoptosis. In oocytes, this postovulatory aging process appears to be similar to that observed in sperm exposed to oxidative stress [29].
Redox homeostasis
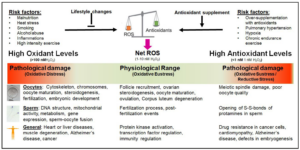
Considering the negative and positive physiological effects of ROS, one needs to understand redox biology. Initially, the concept of “oxidative stress”, i.e. the negative effects of ROS, was introduced into biomedicine by Helmut Sies and was originally defined as “disturbance in the pro-oxidant-antioxidant balance in favour of the former, leading to potential damage” [30]. Later, it was redefined as “an imbalance between oxidants and antioxidants in favour of the oxidants, potentially leading to damage” [31]. On the other hand, it has been shown that an excessive concentration of antioxidants is also causing physiological damage [32-36]. This condition is called “reductive stress” and is as harmful as “oxidative stress” [37]. Taking both sides of the same coin, the negative and the positive effects of ROS, into account, Lushchak and Storey suggest that “oxidative stress is a transient or long-term increase of steady-state ROS levels, disturbing cellular metabolic and signaling pathways, particularly ROS-based ones, and leading to oxidative modifications of an organism’s macromolecules that, if not counterbalanced, may culminate in cell death via necrosis or apoptosis” [38]. This definition recognizes that a fine balance between oxidative stress and reductive stress has to be maintained. Sies defined the physiological range of ROS necessary for normal cellular and bodily functions as “oxidative eustress” [31]. Excessive amounts of antioxidants can trigger numerous diseases including cardiomyopathy, Alzheimer’s disease, dysfunctions of the blood-brain barrier, or infertility [33,35,39-42].
In sperm, oxidative stress is not only causing DNA fragmentation and mitochondrial dysfunction but is also negatively affecting gene expression and sperm-oocyte fusion [43-47]. Furthermore, oxidative stress can affect the genome and epigenome integrity [48,49]. In oocytes, the cytoskeleton, oocyte maturation and fertilization are negatively affected [50,51]. On the other hand, an excessive intake of antioxidants causes an opening of the disulfide bonds of protamines in the sperm head [52,53] and inhibit sperm capacitation [54]. Therefore, for normal reproductive functions of sperm and oocytes, the state of “oxidative eustress” with an approximate cellular H2O2 concentration between 1 nM and 10 nM H2O2 needs to be maintained [55], i.e. the maintenance of the cellular redox homeostasis (Figure 2).
Impact of redox stress on male and female fertility:
Male and female fertility can be compromised by numerous reproductive dysregulations, adverse lifestyle, aging, environmental pollutants or radio- and chemotherapy. In most of these conditions, oxidative stress is significantly involved as a major cause or contributor to the infertility. In men, a major cause of infertility is varicocele. While in the general population, this condition is found in 15% of the adult men, the prevalence is 35% and up to 80% in men with primary and secondary infertility, respectively, much higher [56-59]. Although all of these conditions have different aetiologies, oxidative stress has been implicated as a major mediator for reproductive dysfunctions, thus infertility [60-68].
In sperm, oxidative stress specifically leads to nuclear and mitochondrial DNA decays, including DNA fragmentation [69,70], DNA methylation [71], and telomere changes and attrition [72,73], as well as and mitochondrial DNA damage resulting in mitochondrial dysfunction [74,75]. On the other hand, due to the extraordinary high susceptibility of sperm plasma membranes to oxidative assaults, this leads to lipid peroxidation of membrane lipids, resulting in poor motility, compromised acrosome reaction and the inability of sperm to fuse with the oolemma [76,77], thus poor fertilization ability. Similarly, on the female side, the oocytes, if exposed to excessive oxidative stress, sustain DNA damage including mtDNA damage, altered gene expression, mitochondrial dysfunction, impaired oocyte maturation and luteolysis [78-81].
Sperm health
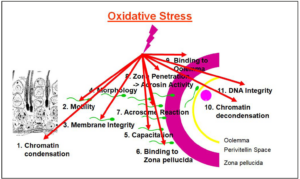
Focusing on sperm health, functions and criteria, numerous studies have shown the detrimental effects of oxidative stress on all sperm functions [82-84], therefore drastically impairing male fertility (Figure 3). On the other hand, one must always consider that normal physiological functions require a small amount of ROS. Panner Selvam et al. [36], by determining the oxidation-reduction potential (ORP) using the MiOXSYS system, showed that good sperm motility, vitality, and normal mitochondrial membrane potential (MMP) depend on a physiological level of ROS [36]. If the redox potential is too high under oxidative stress conditions or too low under reductive stress conditions, sperm will not function properly anymore as indicated by decreased motility, vitality and MMP. The authors also showed that under oxidative and reductive stress conditions, the expression of 3 proteins of the mitochondrial OXPHOS complex, CV-ATPA, CIII-UQCRC2 and CIV-MTCO1 significantly decreased after exposure of sperm to oxidative and reductive stress conditions with CV-ATPA and CIII-UQCRC2 almost disappearing. Essentially, ROS produced in mitochondria of non-capacitating sperm may influence the development of the early embryo by inducing sperm DNA fragmentation [85].
Oocyte and ovarian health
Redox homeostasis is also required for female fertility. Here, ROS are essential for the regulation of the ovarian cycle and ovarian steroidogenesis [87], including oocyte maturation [88], ovulation [89], follicle development, atresia and luteolysis [90,91]. It has also been shown that oxidative stress is intimately involved in ovarian aging, mitochondrial dysfunction, spindle abnormalities, telomere shortening and inflammatory processes such as PCOS or endometriosis [92-95]. In these syndromes, oxidative stress with subsequent mitochondrial dysfunction can affect folliculogenesis, the dialogue between oocyte and cumulus cells, maturation of the ooplasm, chromosome segregation by increasing aneuploidy risks, and cause atresia of granulosa and theca cells. In order to support healthy oocyte development and quality, follicular fluid contains high amounts of various antioxidants [96,97]. Among the antioxidant enzymes, superoxide dismutase [98], catalase [99] and glutathione [100,101] are critical for the maintenance of redox homeostasis for ovulation, the luteal phase, for the protection of genomic integrity, or the development and protection of oocytes.
In follicular fluid of older women, Carbone and co-workers found lower activities of glutathione and catalase together with a lower ratios of catalase/superoxide dismutase and glutathione peroxidase/superoxide dismutase [96]. Age-related oxidative damage as observed as lipid peroxidation, damage to proteins and DNA has also been reported in follicles and ovarian tissue of older women [102,103], thus causing cellular dysfunction, affecting follicle development and consequently oocyte quality [104,105]. This increase in oxidative stress is not only seen in aging women, but also in infertile ones. Zaha et al. [106] reported significantly higher levels of free glutathione in follicular fluid of infertile women as compared to a fertile control group while other oxidative stress markers such as malondialdehyde or superoxide dismutase showed no difference [106]. Also, none of the oxidative stress markers investigated was affected in serum. The higher glutathione levels in follicular fluid of infertile women could possibly be explained as a counter-regulation mechanism against oxidative stress. Furthermore, since oxidative stress is resulting in mitochondrial dysfunction with altered mitochondrial membrane potential, this leads to a release of cytochrome c triggering apoptosis [107]. Since telomeres are very prone to oxidation and due to heterochromatin state of telomeres, these damages are less efficiently repaired [108,109]. A study by Yamada-Fukunaga et al. [110] showed that telomeres in oocytes from older females are significantly shorter.
Effect of carnitines
Aside from eliminating the root cause of oxidative stress, treating it with antioxidants is one of the options. Among the different antioxidants that can neutralize high ROS levels causing oxidative stress is L-carnitine (β-hydroxy-γ-trimethylaminobutyrate), a natural antioxidant that is produced in living organisms from the amino acids lysine and methionine in the liver and kidney. While about 25% of the L-carnitine is synthesized endogenously, the remaining 75% have to be provided by food [7]. L-Carnitine is found in large quantities in red meat, especially mutton and lamb whereas poultry has less L-carnitine and vegetarian foods very little or no L-carnitine.
In human cells, L-carnitine has vital roles in alleviating cellular damage by transferring long-chain fatty acids to the internal mitochondrial membrane for β-oxidation, modulating acyl-Co-A/CoA, reducing the acyl group toxicity by excreting carnitine esters, and having antioxidant and antiradical properties [7,111,112]. L-carnitine improves mitochondrial functions by shuttling fatty acids into β-oxidation, thereby preventing accumulation of damaged lipids [113,114], hence preventing lipid peroxidation of the sensitive sperm membranes. With regard to sperm, oocytes and embryo development, L-carnitine has been shown to improve sperm count, motility, morphology and DNA fragmentation, and regulate oocyte and embryo energetics [115-118] and can therefore improve fertility rates. Treatment of infertile women with L-carnitine resulted in significantly higher transferable embryo rates as well as better quality of embryos [119]. In a systematic review and meta-analysis of 69 studies, Zafar and co-workers reported significantly higher pregnancy rates where men were supplemented with L-carnitine and micronutrients [120]. Similarly, in a randomized, double-blind, placebo-controlled trial including 263 patients, Lahimer et al. [121] found that supplementation of infertile men with L-carnitine and micronutrients significantly improved clinical pregnancy and live birth rates.
Conclusion
In conclusion, mitochondria are the site in eukaryotes where the required energy as well as ROS are produced and are crucial for sperm and oocyte development and function. Although small amounts of ROS are necessary to trigger essential cellular functions, excessive amounts of ROS that are not counteracted by a sufficient amount of antioxidants cause diseases and negatively affect sperm and oocyte functions, hence fertilization and embryo development. Therefore, maintaining redox homeostasis is essential for normal reproductive functions in men and women. In case of oxidative stress due to a number of diseases and health and lifestyle conditions, supplementation with antioxidants can improve the situation. Among these, L-carnitine has shown to be of particular importance as this quaternary amine has not only direct antioxidant properties but is also intimately involved in shuttling long-chain fatty acids into the mitochondrial β-oxidation for energy production as well as the detoxification of damaging lipids.
Acknowledgement
This document was a lecture presented by R.H, at the 15th Congress of the European Society of Gynecology, November 29 to December 2, 2023, Amsterdam, The Netherlands.
Conflicts of interest
The author declares having no conflicts of interest.
Funding
None.